+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human Vault Cage in complex with PARP4 | |||||||||
 Map data Map data | Sharpened cryo-EM map of the human MVP-PARP4 complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Vault / Vault Cage / MVP / Major Vault Protein / SPFH / PARP4 / Poly(ADP-ribose)Polymerase 4 / MINT / Poly(ADP-ribose)Polymerase / Ribonucleoprotein / Megadalton complex / TEP1 / vault RNA / PROTEIN TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology informationprotein activation cascade / Nicotinate metabolism / Maturation of nucleoprotein / ERBB signaling pathway / Maturation of nucleoprotein / NAD+-protein-aspartate ADP-ribosyltransferase activity / NAD+-protein-glutamate ADP-ribosyltransferase activity / NAD+-protein mono-ADP-ribosyltransferase activity / negative regulation of epidermal growth factor receptor signaling pathway / Transferases; Glycosyltransferases; Pentosyltransferases ...protein activation cascade / Nicotinate metabolism / Maturation of nucleoprotein / ERBB signaling pathway / Maturation of nucleoprotein / NAD+-protein-aspartate ADP-ribosyltransferase activity / NAD+-protein-glutamate ADP-ribosyltransferase activity / NAD+-protein mono-ADP-ribosyltransferase activity / negative regulation of epidermal growth factor receptor signaling pathway / Transferases; Glycosyltransferases; Pentosyltransferases / NAD+ poly-ADP-ribosyltransferase activity / mRNA transport / nuclear pore / nucleotidyltransferase activity / spindle microtubule / protein modification process / protein transport / secretory granule lumen / protein phosphatase binding / ficolin-1-rich granule lumen / cytoskeleton / cell population proliferation / intracellular signal transduction / response to xenobiotic stimulus / inflammatory response / ribonucleoprotein complex / DNA repair / DNA damage response / Neutrophil degranulation / protein kinase binding / perinuclear region of cytoplasm / enzyme binding / DNA binding / extracellular exosome / extracellular region / nucleoplasm / identical protein binding / membrane / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Lodwick JE / Zhao M | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2009 Journal: Science / Year: 2009Title: The structure of rat liver vault at 3.5 angstrom resolution. Authors: Hideaki Tanaka / Koji Kato / Eiki Yamashita / Tomoyuki Sumizawa / Yong Zhou / Min Yao / Kenji Iwasaki / Masato Yoshimura / Tomitake Tsukihara /  Abstract: Vaults are among the largest cytoplasmic ribonucleoprotein particles and are found in numerous eukaryotic species. Roles in multidrug resistance and innate immunity have been suggested, but the ...Vaults are among the largest cytoplasmic ribonucleoprotein particles and are found in numerous eukaryotic species. Roles in multidrug resistance and innate immunity have been suggested, but the cellular function remains unclear. We have determined the x-ray structure of rat liver vault at 3.5 angstrom resolution and show that the cage structure consists of a dimer of half-vaults, with each half-vault comprising 39 identical major vault protein (MVP) chains. Each MVP monomer folds into 12 domains: nine structural repeat domains, a shoulder domain, a cap-helix domain, and a cap-ring domain. Interactions between the 42-turn-long cap-helix domains are key to stabilizing the particle. The shoulder domain is structurally similar to a core domain of stomatin, a lipid-raft component in erythrocytes and epithelial cells. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_44955.map.gz emd_44955.map.gz | 1.8 GB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-44955-v30.xml emd-44955-v30.xml emd-44955.xml emd-44955.xml | 32.3 KB 32.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_44955_fsc.xml emd_44955_fsc.xml | 26.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_44955.png emd_44955.png | 140.5 KB | ||
| Filedesc metadata |  emd-44955.cif.gz emd-44955.cif.gz | 9.5 KB | ||
| Others |  emd_44955_additional_1.map.gz emd_44955_additional_1.map.gz emd_44955_half_map_1.map.gz emd_44955_half_map_1.map.gz emd_44955_half_map_2.map.gz emd_44955_half_map_2.map.gz | 979.6 MB 1.8 GB 1.8 GB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44955 http://ftp.pdbj.org/pub/emdb/structures/EMD-44955 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44955 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44955 | HTTPS FTP |
-Related structure data
| Related structure data |  9bw6MC  9bw5C  9bw7C  9mxjC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_44955.map.gz / Format: CCP4 / Size: 1.9 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_44955.map.gz / Format: CCP4 / Size: 1.9 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened cryo-EM map of the human MVP-PARP4 complex | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Unsharpened cryo-EM map of the human MVP-PARP4 complex
| File | emd_44955_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened cryo-EM map of the human MVP-PARP4 complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
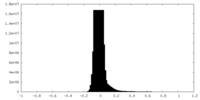
| Density Histograms |
-Half map: Cryo-EM half map of the human MVP-PARP4 complex
| File | emd_44955_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM half map of the human MVP-PARP4 complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
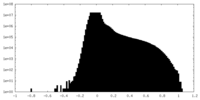
| Density Histograms |
-Half map: Cryo-EM half map of the human MVP-PARP4 complex
| File | emd_44955_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM half map of the human MVP-PARP4 complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
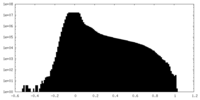
| Density Histograms |
- Sample components
Sample components
-Entire : Binary complex of human PARP4 bound to oligomerized human MVP
| Entire | Name: Binary complex of human PARP4 bound to oligomerized human MVP |
|---|---|
| Components |
|
-Supramolecule #1: Binary complex of human PARP4 bound to oligomerized human MVP
| Supramolecule | Name: Binary complex of human PARP4 bound to oligomerized human MVP type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.7 MDa |
-Macromolecule #1: Major vault protein
| Macromolecule | Name: Major vault protein / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 99.452766 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MATEEFIIRI PPYHYIHVLD QNSNVSRVEV GPKTYIRQDN ERVLFAPMRM VTVPPRHYCT VANPVSRDAQ GLVLFDVTGQ VRLRHADLE IRLAQDPFPL YPGEVLEKDI TPLQVVLPNT ALHLKALLDF EDKDGDKVVA GDEWLFEGPG TYIPRKEVEV V EIIQATII ...String: MATEEFIIRI PPYHYIHVLD QNSNVSRVEV GPKTYIRQDN ERVLFAPMRM VTVPPRHYCT VANPVSRDAQ GLVLFDVTGQ VRLRHADLE IRLAQDPFPL YPGEVLEKDI TPLQVVLPNT ALHLKALLDF EDKDGDKVVA GDEWLFEGPG TYIPRKEVEV V EIIQATII RQNQALRLRA RKECWDRDGK ERVTGEEWLV TTVGAYLPAV FEEVLDLVDA VILTEKTALH LRARRNFRDF RG VSRRTGE EWLVTVQDTE AHVPDVHEEV LGVVPITTLG PHNYCVILDP VGPDGKNQLG QKRVVKGEKS FFLQPGEQLE QGI QDVYVL SEQQGLLLRA LQPLEEGEDE EKVSHQAGDH WLIRGPLEYV PSAKVEVVEE RQAIPLDENE GIYVQDVKTG KVRA VIGST YMLTQDEVLW EKELPPGVEE LLNKGQDPLA DRGEKDTAKS LQPLAPRNKT RVVSYRVPHN AAVQVYDYRE KRARV VFGP ELVSLGPEEQ FTVLSLSAGR PKRPHARRAL CLLLGPDFFT DVITIETADH ARLQLQLAYN WHFEVNDRKD PQETAK LFS VPDFVGDACK AIASRVRGAV ASVTFDDFHK NSARIIRTAV FGFETSEAKG PDGMALPRPR DQAVFPQNGL VVSSVDV QS VEPVDQRTRD ALQRSVQLAI EITTNSQEAA AKHEAQRLEQ EARGRLERQK ILDQSEAEKA RKELLELEAL SMAVESTG T AKAEAESRAE AARIEGEGSV LQAKLKAQAL AIETEAELQR VQKVRELELV YARAQLELEV SKAQQLAEVE VKKFKQMTE AIGPSTIRDL AVAGPEMQVK LLQSLGLKST LITDGSTPIN LFNTAFGLLG MGPEGQPLGR RVASGPSPGE GISPQSAQAP QAPGDNHVV PVLR UniProtKB: Major vault protein |
-Macromolecule #2: Protein mono-ADP-ribosyltransferase PARP4
| Macromolecule | Name: Protein mono-ADP-ribosyltransferase PARP4 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO EC number: Transferases; Glycosyltransferases; Pentosyltransferases |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 192.810172 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MVMGIFANCI FCLKVKYLPQ QQKKKLQTDI KENGGKFSFS LNPQCTHIIL DNADVLSQYQ LNSIQKNHVH IANPDFIWKS IREKRLLDV KNYDPYKPLD ITPPPDQKAS SSEVKTEGLC PDSATEEEDT VELTEFGMQN VEIPHLPQDF EVAKYNTLEK V GMEGGQEA ...String: MVMGIFANCI FCLKVKYLPQ QQKKKLQTDI KENGGKFSFS LNPQCTHIIL DNADVLSQYQ LNSIQKNHVH IANPDFIWKS IREKRLLDV KNYDPYKPLD ITPPPDQKAS SSEVKTEGLC PDSATEEEDT VELTEFGMQN VEIPHLPQDF EVAKYNTLEK V GMEGGQEA VVVELQCSRD SRDCPFLISS HFLLDDGMET RRQFAIKKTS EDASEYFENY IEELKKQGFL LREHFTPEAT QL ASEQLQA LLLEEVMNSS TLSQEVSDLV EMIWAEALGH LEHMLLKPVN RISLNDVSKA EGILLLVKAA LKNGETAEQL QKM MTEFYR LIPHKGTMPK EVNLGLLAKK ADLCQLIRDM VNVCETNLSK PNPPSLAKYR ALRCKIEHVE QNTEEFLRVR KEVL QNHHS KSPVDVLQIF RVGRVNETTE FLSKLGNVRP LLHGSPVQNI VGILCRGLLL PKVVEDRGVQ RTDVGNLGSG IYFSD SLST SIKYSHPGET DGTRLLLICD VALGKCMDLH EKDFSLTEAP PGYDSVHGVS QTASVTTDFE DDEFVVYKTN QVKMKY IIK FSMPGDQIKD FHPSDHTELE EYRPEFSNFS KVEDYQLPDA KTSSSTKAGL QDASGNLVPL EDVHIKGRII DTVAQVI VF QTYTNKSHVP IEAKYIFPLD DKAAVCGFEA FINGKHIVGE IKEKEEAQQE YLEAVTQGHG AYLMSQDAPD VFTVSVGN L PPKAKVLIKI TYITELSILG TVGVFFMPAT VAPWQQDKAL NENLQDTVEK ICIKEIGTKQ SFSLTMSIEM PYVIEFIFS DTHELKQKRT DCKAVISTME GSSLDSSGFS LHIGLSAAYL PRMWVEKHPE KESEACMLVF QPDLDVDLPD LASESEVIIC LDCSSSMEG VTFLQAKQIA LHALSLVGEK QKVNIIQFGT GYKELFSYPK HITSNTMAAE FIMSATPTMG NTDFWKTLRY L SLLYPARG SRNILLVSDG HLQDESLTLQ LVKRSRPHTR LFACGIGSTA NRHVLRILSQ CGAGVFEYFN AKSKHSWRKQ IE DQMTRLC SPSCHSVSVK WQQLNPDVPE ALQAPAQVPS LFLNDRLLVY GFIPHCTQAT LCALIQEKEF RTMVSTTELQ KTT GTMIHK LAARALIRDY EDGILHENET SHEMKKQTLK SLIIKLSKEN SLITQFTSFV AVEKRDENES PFPDIPKVSE LIAK EDVDF LPYMSWQGEP QEAVRNQSLL ASSEWPELRL SKRKHRKIPF SKRKMELSQP EVSEDFEEDG LGVLPAFTSN LERGG VEKL LDLSWTESCK PTATEPLFKK VSPWETSTSS FFPILAPAVG SYLPPTARAH SPASLSFASY RQVASFGSAA PPRQFD ASQ FSQGPVPGTC ADWIPQSASC PTGPPQNPPS SPYCGIVFSG SSLSSAQSAP LQHPGGFTTR PSAGTFPELD SPQLHFS LP TDPDPIRGFG SYHPSASSPF HFQPSAASLT ANLRLPMASA LPEALCSQSR TTPVDLCLLE ESVGSLEGSR CPVFAFQS S DTESDELSEV LQDSCFLQIK CDTKDDSILC FLEVKEEDEI VCIQHWQDAV PWTELLSLQT EDGFWKLTPE LGLILNLNT NGLHSFLKQK GIQSLGVKGR ECLLDLIATM LVLQFIRTRL EKEGIVFKSL MKMDDASISR NIPWAFEAIK QASEWVRRTE GQYPSICPR LELGNDWDSA TKQLLGLQPI STVSPLHRVL HYSQG UniProtKB: Protein mono-ADP-ribosyltransferase PARP4 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: 50 mM HEPES, 5 mM MgCl2, 5 mM CaCl2, 0.25 mM DTT | |||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 14 / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 15 sec. / Pretreatment - Atmosphere: OTHER Details: Quantifoil grids with a 2 nm continuous carbon coating were subjected to a 15 s, 5W plasma cleaning program in O2 gas | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV Details: Vitrification carried out under standard conditions. | |||||||||||||||
| Details | Sample was monodisperse |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Spherical aberration corrector: Microscope was modified with a Cs corrector with two hexapole elements Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 8 eV |
| Details | Preliminary grid screening was performed manually. |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number real images: 7491 / Average electron dose: 45.0 e/Å2 Details: Images were collected in movie mode, with 40 frames per image. Data was collected in SerialEM using a strategy of 3 by 3 + 1 shots per hole. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.01 mm / Nominal defocus max: 1.7 µm / Nominal defocus min: 0.7000000000000001 µm / Nominal magnification: 53000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Residue range: 1-845 / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Details | The starting model was the rat homologue of MVP from a previous crystal structure of the vault cage (PDB accession no 4V60). The final model was refined in real space and validated using PHENIX. |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
| Output model |  PDB-9bw6: |
-Atomic model buiding 2
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Residue range: 1562-1724 / Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Details | PARP4's MINT domain, predicted by AlphaFold2, was used as the starting model. The final model was refined in real space and validated using PHENIX. |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
| Output model |  PDB-9bw6: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)