+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cryo-EM structure of human HGSNAT bound with CoA and product analog | |||||||||
 マップデータ マップデータ | sharpened map | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | Membrane protein / Lysosome / Transmembrane acetyltransferase / CoA / product / Heperan sulfate / TRANSFERASE-INHIBITOR complex | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報heparan-alpha-glucosaminide N-acetyltransferase / heparan-alpha-glucosaminide N-acetyltransferase activity / MPS IIIC - Sanfilippo syndrome C / heparan sulfate proteoglycan catabolic process / HS-GAG degradation / lysosomal transport / acyltransferase activity / protein complex oligomerization / tertiary granule membrane / specific granule membrane ...heparan-alpha-glucosaminide N-acetyltransferase / heparan-alpha-glucosaminide N-acetyltransferase activity / MPS IIIC - Sanfilippo syndrome C / heparan sulfate proteoglycan catabolic process / HS-GAG degradation / lysosomal transport / acyltransferase activity / protein complex oligomerization / tertiary granule membrane / specific granule membrane / lysosomal lumen / lysosomal membrane / Neutrophil degranulation / plasma membrane 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.2 Å | |||||||||
 データ登録者 データ登録者 | Li F / Zhao B | |||||||||
| 資金援助 |  米国, 2件 米国, 2件
| |||||||||
 引用 引用 |  ジャーナル: Nat Commun / 年: 2024 ジャーナル: Nat Commun / 年: 2024タイトル: Structural and mechanistic insights into a lysosomal membrane enzyme HGSNAT involved in Sanfilippo syndrome. 著者: Boyang Zhao / Zhongzheng Cao / Yi Zheng / Phuong Nguyen / Alisa Bowen / Robert H Edwards / Robert M Stroud / Yi Zhou / Menno Van Lookeren Campagne / Fei Li /  要旨: Heparan sulfate (HS) is degraded in lysosome by a series of glycosidases. Before the glycosidases can act, the terminal glucosamine of HS must be acetylated by the integral lysosomal membrane enzyme ...Heparan sulfate (HS) is degraded in lysosome by a series of glycosidases. Before the glycosidases can act, the terminal glucosamine of HS must be acetylated by the integral lysosomal membrane enzyme heparan-α-glucosaminide N-acetyltransferase (HGSNAT). Mutations of HGSNAT cause HS accumulation and consequently mucopolysaccharidosis IIIC, a devastating lysosomal storage disease characterized by progressive neurological deterioration and early death where no treatment is available. HGSNAT catalyzes a unique transmembrane acetylation reaction where the acetyl group of cytosolic acetyl-CoA is transported across the lysosomal membrane and attached to HS in one reaction. However, the reaction mechanism remains elusive. Here we report six cryo-EM structures of HGSNAT along the reaction pathway. These structures reveal a dimer arrangement and a unique structural fold, which enables the elucidation of the reaction mechanism. We find that a central pore within each monomer traverses the membrane and controls access of cytosolic acetyl-CoA to the active site at its luminal mouth where glucosamine binds. A histidine-aspartic acid catalytic dyad catalyzes the transfer reaction via a ternary complex mechanism. Furthermore, the structures allow the mapping of disease-causing variants and reveal their potential impact on the function, thus creating a framework to guide structure-based drug discovery efforts. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_43339.map.gz emd_43339.map.gz | 290.4 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-43339-v30.xml emd-43339-v30.xml emd-43339.xml emd-43339.xml | 17.7 KB 17.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
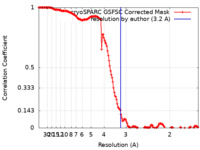
| FSC (解像度算出) |  emd_43339_fsc.xml emd_43339_fsc.xml | 14.3 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_43339.png emd_43339.png | 85.6 KB | ||
| Filedesc metadata |  emd-43339.cif.gz emd-43339.cif.gz | 6.4 KB | ||
| その他 |  emd_43339_additional_1.map.gz emd_43339_additional_1.map.gz emd_43339_half_map_1.map.gz emd_43339_half_map_1.map.gz emd_43339_half_map_2.map.gz emd_43339_half_map_2.map.gz | 153.4 MB 285.2 MB 285.2 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43339 http://ftp.pdbj.org/pub/emdb/structures/EMD-43339 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43339 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43339 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_43339_validation.pdf.gz emd_43339_validation.pdf.gz | 859.1 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_43339_full_validation.pdf.gz emd_43339_full_validation.pdf.gz | 858.7 KB | 表示 | |
| XML形式データ |  emd_43339_validation.xml.gz emd_43339_validation.xml.gz | 23.3 KB | 表示 | |
| CIF形式データ |  emd_43339_validation.cif.gz emd_43339_validation.cif.gz | 30.4 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43339 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43339 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43339 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43339 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_43339.map.gz / 形式: CCP4 / 大きさ: 307.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_43339.map.gz / 形式: CCP4 / 大きさ: 307.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | sharpened map | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.813 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-追加マップ: unsharpened map used to dock TM1 (residue 191-226)....
| ファイル | emd_43339_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | unsharpened map used to dock TM1 (residue 191-226). All other areas are built based on the sharpened map. The entire model is refined against the sharpened map. | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: half map
| ファイル | emd_43339_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | half map | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: half map
| ファイル | emd_43339_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | half map | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Purified human HGSNAT
| 全体 | 名称: Purified human HGSNAT |
|---|---|
| 要素 |
|
-超分子 #1: Purified human HGSNAT
| 超分子 | 名称: Purified human HGSNAT / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 73 KDa |
-分子 #1: Heparan-alpha-glucosaminide N-acetyltransferase
| 分子 | 名称: Heparan-alpha-glucosaminide N-acetyltransferase / タイプ: protein_or_peptide / ID: 1 / コピー数: 2 / 光学異性体: LEVO / EC番号: heparan-alpha-glucosaminide N-acetyltransferase |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 73.365039 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: MTGARASAAE QRRAGRSGQA RAAERAAGMS GAGRALAALL LAASVLSAAL LAPGGSSGRD AQAAPPRDLD KKRHAELKMD QALLLIHNE LLWTNLTVYW KSECCYHCLF QVLVNVPQSP KAGKPSAAAA SVSTQHGSIL QLNDTLEEKE VCRLEYRFGE F GNYSLLVK ...文字列: MTGARASAAE QRRAGRSGQA RAAERAAGMS GAGRALAALL LAASVLSAAL LAPGGSSGRD AQAAPPRDLD KKRHAELKMD QALLLIHNE LLWTNLTVYW KSECCYHCLF QVLVNVPQSP KAGKPSAAAA SVSTQHGSIL QLNDTLEEKE VCRLEYRFGE F GNYSLLVK NIHNGVSEIA CDLAVNEDPV DSNLPVSIAF LIGLAVIIVI SFLRLLLSLD DFNNWISKAI SSRETDRLIN SE LGSPSRT DPLDGDVQPA TWRLSALPPR LRSVDTFRGI ALILMVFVNY GGGKYWYFKH ASWNGLTVAD LVFPWFVFIM GSS IFLSMT SILQRGCSKF RLLGKIAWRS FLLICIGIII VNPNYCLGPL SWDKVRIPGV LQRLGVTYFV VAVLELLFAK PVPE HCASE RSCLSLRDIT SSWPQWLLIL VLEGLWLGLT FLLPVPGCPT GYLGPGGIGD FGKYPNCTGG AAGYIDRLLL GDDHL YQHP SSAVLYHTEV AYDPEGILGT INSIVMAFLG VQAGKILLYY KARTKDILIR FTAWCCILGL ISVALTKVSE NEGFIP VNK NLWSLSYVTT LSSFAFFILL VLYPVVDVKG LWTGTPFFYP GMNSILVYVG HEVFENYFPF QWKLKDNQSH KEHLTQN IV ATALWVLIAY ILYRKKIFWK I UniProtKB: Heparan-alpha-glucosaminide N-acetyltransferase |
-分子 #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| 分子 | 名称: 2-acetamido-2-deoxy-beta-D-glucopyranose / タイプ: ligand / ID: 3 / コピー数: 5 / 式: NAG |
|---|---|
| 分子量 | 理論値: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-分子 #4: COENZYME A
| 分子 | 名称: COENZYME A / タイプ: ligand / ID: 4 / コピー数: 2 / 式: COA |
|---|---|
| 分子量 | 理論値: 767.534 Da |
| Chemical component information |  ChemComp-COA: |
-分子 #5: 4-methyl-2-oxo-2H-1-benzopyran-7-yl 2-acetamido-2-deoxy-beta-D-gl...
| 分子 | 名称: 4-methyl-2-oxo-2H-1-benzopyran-7-yl 2-acetamido-2-deoxy-beta-D-glucopyranoside タイプ: ligand / ID: 5 / コピー数: 2 / 式: A1AC0 |
|---|---|
| 分子量 | 理論値: 379.361 Da |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 4.4 mg/mL |
|---|---|
| 緩衝液 | pH: 7.5 |
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) 平均電子線量: 1.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 22.0 µm / 最小 デフォーカス(公称値): 10.0 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)