[English] 日本語
 Yorodumi
Yorodumi- EMDB-4330: Structure of a partial yeast 48S preinitiation complex with eIF5 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4330 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of a partial yeast 48S preinitiation complex with eIF5 N-terminal domain (Map A) | |||||||||
 Map data Map data | For optimal visualization of all tRNA, eIF2 gamma and eIF3 PCI domains, gauss-filter the map by 1.1 and display it at 0.02 contour level. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Kluyveromyces lactis (yeast) Kluyveromyces lactis (yeast) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.53 Å | |||||||||
 Authors Authors | Llacer JL / Hussain T / Gordiyenko Y / Ramakrishnan V | |||||||||
 Citation Citation |  Journal: Elife / Year: 2018 Journal: Elife / Year: 2018Title: Translational initiation factor eIF5 replaces eIF1 on the 40S ribosomal subunit to promote start-codon recognition. Authors: Jose Luis Llácer / Tanweer Hussain / Adesh K Saini / Jagpreet Singh Nanda / Sukhvir Kaur / Yuliya Gordiyenko / Rakesh Kumar / Alan G Hinnebusch / Jon R Lorsch / V Ramakrishnan /     Abstract: In eukaryotic translation initiation, AUG recognition of the mRNA requires accommodation of Met-tRNA in a 'P' state, which is antagonized by the factor eIF1. eIF5 is a GTPase activating protein (GAP) ...In eukaryotic translation initiation, AUG recognition of the mRNA requires accommodation of Met-tRNA in a 'P' state, which is antagonized by the factor eIF1. eIF5 is a GTPase activating protein (GAP) of eIF2 that additionally promotes stringent AUG selection, but the molecular basis of its dual function was unknown. We present a cryo-electron microscopy (cryo-EM) reconstruction of a yeast 48S pre-initiation complex (PIC), at an overall resolution of 3.0 Å, featuring the N-terminal domain (NTD) of eIF5 bound to the 40S subunit at the location vacated by eIF1. eIF5 interacts with and allows a more accommodated orientation of Met-tRNA. Substitutions of eIF5 residues involved in the eIF5-NTD/tRNA interaction influenced initiation at near-cognate UUG codons and the closed/open PIC conformation in vitro, consistent with direct stabilization of the codon:anticodon duplex by the wild-type eIF5-NTD. The present structure reveals the basis for a key role of eIF5 in start-codon selection. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4330.map.gz emd_4330.map.gz | 94.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4330-v30.xml emd-4330-v30.xml emd-4330.xml emd-4330.xml | 24.5 KB 24.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_4330.png emd_4330.png | 222.4 KB | ||
| Others |  emd_4330_half_map_1.map.gz emd_4330_half_map_1.map.gz emd_4330_half_map_2.map.gz emd_4330_half_map_2.map.gz | 90.6 MB 90.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4330 http://ftp.pdbj.org/pub/emdb/structures/EMD-4330 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4330 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4330 | HTTPS FTP |
-Related structure data
| Related structure data |  4327C  4328C  4329C  4331C  6fyxC  6fyyC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4330.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4330.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | For optimal visualization of all tRNA, eIF2 gamma and eIF3 PCI domains, gauss-filter the map by 1.1 and display it at 0.02 contour level. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.34 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
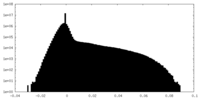
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: #1
| File | emd_4330_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
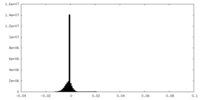
| Density Histograms |
-Half map: #2
| File | emd_4330_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
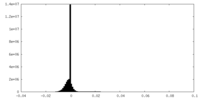
| Density Histograms |
- Sample components
Sample components
-Entire : Structure of a partial yeast 48S preinitiation complex with eIF5 ...
| Entire | Name: Structure of a partial yeast 48S preinitiation complex with eIF5 N-terminal domain (model A) |
|---|---|
| Components |
|
-Supramolecule #1: Structure of a partial yeast 48S preinitiation complex with eIF5 ...
| Supramolecule | Name: Structure of a partial yeast 48S preinitiation complex with eIF5 N-terminal domain (model A) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#47 |
|---|---|
| Source (natural) | Organism:  Kluyveromyces lactis (yeast) Kluyveromyces lactis (yeast) |
| Molecular weight | Theoretical: 1.8 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 6.5 Component:
| ||||||||||||||||||
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK I |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Temperature | Min: 90.0 K / Max: 100.0 K |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Number grids imaged: 3 / Number real images: 3600 / Average exposure time: 1.1 sec. / Average electron dose: 40.0 e/Å2 Details: Images were collected in movie-mode at 32 frames per second |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 104478 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 78000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: RECIPROCAL / Protocol: OTHER / Overall B value: 66 / Target criteria: FSC |
|---|
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)