[English] 日本語
 Yorodumi
Yorodumi- EMDB-42595: Structure of the Measles virus Fusion protein in the pre-fusion c... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the Measles virus Fusion protein in the pre-fusion conformation with bound [FIP-HRC]2-PEG11 | ||||||||||||||||||
 Map data Map data | map sharp | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | VIRAL PROTEIN / glycoprotein / immune system / measles / high-resolution / ectodomain / post-fusion / small molecule / inhibitor / complex | ||||||||||||||||||
| Function / homology | Precursor fusion glycoprotein F0, Paramyxoviridae / Fusion glycoprotein F0 / fusion of virus membrane with host plasma membrane / viral envelope / symbiont entry into host cell / host cell plasma membrane / virion membrane / membrane / Fusion glycoprotein F0 Function and homology information Function and homology information | ||||||||||||||||||
| Biological species |  Measles virus strain Ichinose-B95a / synthetic construct (others) Measles virus strain Ichinose-B95a / synthetic construct (others) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.34 Å | ||||||||||||||||||
 Authors Authors | Zyla D / Saphire EO | ||||||||||||||||||
| Funding support |  Switzerland, Switzerland,  United States, 5 items United States, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Science / Year: 2024 Journal: Science / Year: 2024Title: A neutralizing antibody prevents postfusion transition of measles virus fusion protein. Authors: Dawid S Zyla / Roberta Della Marca / Gele Niemeyer / Gillian Zipursky / Kyle Stearns / Cameron Leedale / Elizabeth B Sobolik / Heather M Callaway / Chitra Hariharan / Weiwei Peng / Diptiben ...Authors: Dawid S Zyla / Roberta Della Marca / Gele Niemeyer / Gillian Zipursky / Kyle Stearns / Cameron Leedale / Elizabeth B Sobolik / Heather M Callaway / Chitra Hariharan / Weiwei Peng / Diptiben Parekh / Tara C Marcink / Ruben Diaz Avalos / Branka Horvat / Cyrille Mathieu / Joost Snijder / Alexander L Greninger / Kathryn M Hastie / Stefan Niewiesk / Anne Moscona / Matteo Porotto / Erica Ollmann Saphire /      Abstract: Measles virus (MeV) presents a public health threat that is escalating as vaccine coverage in the general population declines and as populations of immunocompromised individuals, who cannot be ...Measles virus (MeV) presents a public health threat that is escalating as vaccine coverage in the general population declines and as populations of immunocompromised individuals, who cannot be vaccinated, increase. There are no approved therapeutics for MeV. Neutralizing antibodies targeting viral fusion are one potential therapeutic approach but have not yet been structurally characterized or advanced to clinical use. We present cryo-electron microscopy (cryo-EM) structures of prefusion F alone [2.1-angstrom (Å) resolution], F complexed with a fusion-inhibitory peptide (2.3-Å resolution), F complexed with the neutralizing and protective monoclonal antibody (mAb) 77 (2.6-Å resolution), and an additional structure of postfusion F (2.7-Å resolution). In vitro assays and examination of additional EM classes show that mAb 77 binds prefusion F, arrests F in an intermediate state, and prevents transition to the postfusion conformation. These structures shed light on antibody-mediated neutralization that involves arrest of fusion proteins in an intermediate state. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42595.map.gz emd_42595.map.gz | 97.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42595-v30.xml emd-42595-v30.xml emd-42595.xml emd-42595.xml | 22.2 KB 22.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_42595.png emd_42595.png | 73 KB | ||
| Masks |  emd_42595_msk_1.map emd_42595_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-42595.cif.gz emd-42595.cif.gz | 7.1 KB | ||
| Others |  emd_42595_half_map_1.map.gz emd_42595_half_map_1.map.gz emd_42595_half_map_2.map.gz emd_42595_half_map_2.map.gz | 95.5 MB 95.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42595 http://ftp.pdbj.org/pub/emdb/structures/EMD-42595 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42595 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42595 | HTTPS FTP |
-Related structure data
| Related structure data |  8uuqMC  8ut2C  8utfC  8uupC  9at8C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42595.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42595.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map sharp | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.88 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_42595_msk_1.map emd_42595_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
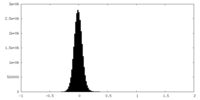
| Density Histograms |
-Half map: half 1
| File | emd_42595_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
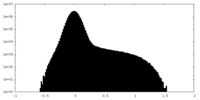
| Density Histograms |
-Half map: half 2
| File | emd_42595_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Structure of the Measles virus Fusion protein in the pre-fusion c...
| Entire | Name: Structure of the Measles virus Fusion protein in the pre-fusion conformation with bound [FIP-HRC]2-PEG11 |
|---|---|
| Components |
|
-Supramolecule #1: Structure of the Measles virus Fusion protein in the pre-fusion c...
| Supramolecule | Name: Structure of the Measles virus Fusion protein in the pre-fusion conformation with bound [FIP-HRC]2-PEG11 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Measles virus strain Ichinose-B95a / Strain: Ichinose-B95a Measles virus strain Ichinose-B95a / Strain: Ichinose-B95a |
-Macromolecule #1: Fusion glycoprotein F0
| Macromolecule | Name: Fusion glycoprotein F0 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Measles virus strain Ichinose-B95a Measles virus strain Ichinose-B95a |
| Molecular weight | Theoretical: 12.498768 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGLKVNVSAI FMAVLLTLQT PTGQIHWGNL SKIGVVGIGS ASYKVMTRSS HQSLVIKLMP NITLLNNCTR VEIAEYRRLL RTVLEPIRD ALNAMTQNIR PVQSVASSRR HKR UniProtKB: Fusion glycoprotein F0 |
-Macromolecule #2: Fusion glycoprotein F0
| Macromolecule | Name: Fusion glycoprotein F0 / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Measles virus strain Ichinose-B95a Measles virus strain Ichinose-B95a |
| Molecular weight | Theoretical: 44.89882 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: FAGVVLAGAA LGVATAAQIT AGIALHQSML NSQAIDNLRA SLETTNQAIE AIRQAGQGMI LAVQGVQDYI NNELIPSMNQ LSCDLIGQK LGLKLLRYYT EILSLFGPSL RDPISAEISI QALSYALGGD INKVLEKLGY SGGDLLGILE SRGIKARITH V DTESYFIV ...String: FAGVVLAGAA LGVATAAQIT AGIALHQSML NSQAIDNLRA SLETTNQAIE AIRQAGQGMI LAVQGVQDYI NNELIPSMNQ LSCDLIGQK LGLKLLRYYT EILSLFGPSL RDPISAEISI QALSYALGGD INKVLEKLGY SGGDLLGILE SRGIKARITH V DTESYFIV LSIAYPTLSE IKGVIVHRLE GVSYNIGSQE WYTTVPKYVA TQGYLISNFD ESSCTFMPEG TVCSQNALYP MS PLLQECL RGSTKSCART LVSGSFGNRF ILSQGNLIAN CASILCKCYT TGTIINQDPD KILTYIAADH CPVVEVNGVT IQV GSRRYP DAVYLHRIDL GPPISLGRLD VGTNLGNAIA KLEDAKELLE SSDQILRSMK GLSSTSIGVD DDDKAGWSHP QFEK GGGSG GGSGGGSWSH PQFEK UniProtKB: Fusion glycoprotein F0 |
-Macromolecule #3: [FIP-HRC]2-PEG11
| Macromolecule | Name: [FIP-HRC]2-PEG11 / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 503.546 Da |
| Sequence | String: (P6S)FFG |
-Macromolecule #6: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 6 / Number of copies: 3 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #7: water
| Macromolecule | Name: water / type: ligand / ID: 7 / Number of copies: 33 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.38 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: HEPES 50 mM, pH 8.0, NaCl 150 mM |
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 400 / Support film - Material: GRAPHENE OXIDE / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 3 / Number real images: 10716 / Average exposure time: 2.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)