[English] 日本語
 Yorodumi
Yorodumi- EMDB-41495: Crosslinked 6-deoxyerythronolide B synthase (DEBS) Module 3 in co... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crosslinked 6-deoxyerythronolide B synthase (DEBS) Module 3 in complex with antibody fragment 1B2: cis-oriented 1B2 and ACP | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | polyketide synthase / antibody / BIOSYNTHETIC PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology information6-deoxyerythronolide-B synthase / erythronolide synthase activity / macrolide biosynthetic process / fatty acid synthase activity / phosphopantetheine binding / 3-oxoacyl-[acyl-carrier-protein] synthase activity / fatty acid biosynthetic process / oxidoreductase activity Similarity search - Function | |||||||||||||||
| Biological species |  Saccharopolyspora erythraea (bacteria) / Saccharopolyspora erythraea (bacteria) /  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.46 Å | |||||||||||||||
 Authors Authors | Cogan DP / Soohoo AM / Chen M / Brodsky KL / Liu Y / Khosla C | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2025 Journal: Nat Chem Biol / Year: 2025Title: Structural basis for intermodular communication in assembly-line polyketide biosynthesis. Authors: Dillon P Cogan / Alexander M Soohoo / Muyuan Chen / Yan Liu / Krystal L Brodsky / Chaitan Khosla /  Abstract: Assembly-line polyketide synthases (PKSs) are modular multi-enzyme systems with considerable potential for genetic reprogramming. Understanding how they selectively transport biosynthetic ...Assembly-line polyketide synthases (PKSs) are modular multi-enzyme systems with considerable potential for genetic reprogramming. Understanding how they selectively transport biosynthetic intermediates along a defined sequence of active sites could be harnessed to rationally alter PKS product structures. To investigate functional interactions between PKS catalytic and substrate acyl carrier protein (ACP) domains, we employed a bifunctional reagent to crosslink transient domain-domain interfaces of a prototypical assembly line, the 6-deoxyerythronolide B synthase, and resolved their structures by single-particle cryogenic electron microscopy (cryo-EM). Together with statistical per-particle image analysis of cryo-EM data, we uncovered interactions between ketosynthase (KS) and ACP domains that discriminate between intra-modular and inter-modular communication while reinforcing the relevance of conformational asymmetry during the catalytic cycle. Our findings provide a foundation for the structure-based design of hybrid PKSs comprising biosynthetic modules from different naturally occurring assembly lines. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41495.map.gz emd_41495.map.gz | 172.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41495-v30.xml emd-41495-v30.xml emd-41495.xml emd-41495.xml | 24.8 KB 24.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41495.png emd_41495.png | 24.5 KB | ||
| Filedesc metadata |  emd-41495.cif.gz emd-41495.cif.gz | 8.3 KB | ||
| Others |  emd_41495_half_map_1.map.gz emd_41495_half_map_1.map.gz emd_41495_half_map_2.map.gz emd_41495_half_map_2.map.gz | 318 MB 318 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41495 http://ftp.pdbj.org/pub/emdb/structures/EMD-41495 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41495 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41495 | HTTPS FTP |
-Related structure data
| Related structure data |  8tpwMC  8tjnC  8tjoC  8tjpC  8tkoC  8tpxC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41495.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41495.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.257 Å | ||||||||||||||||||||||||||||||||||||
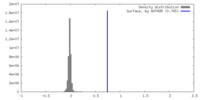
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_41495_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
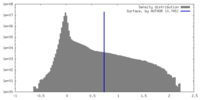
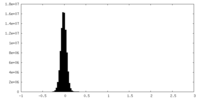
| Density Histograms |
-Half map: #2
| File | emd_41495_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
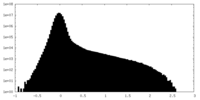
| Density Histograms |
- Sample components
Sample components
-Entire : Crosslinked DEBS Module 3 in complex with Antibody Fragment 1B2
| Entire | Name: Crosslinked DEBS Module 3 in complex with Antibody Fragment 1B2 |
|---|---|
| Components |
|
-Supramolecule #1: Crosslinked DEBS Module 3 in complex with Antibody Fragment 1B2
| Supramolecule | Name: Crosslinked DEBS Module 3 in complex with Antibody Fragment 1B2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Saccharopolyspora erythraea (bacteria) Saccharopolyspora erythraea (bacteria) |
| Molecular weight | Theoretical: 420 KDa |
-Macromolecule #1: EryAII,EryAII,EryAII,EryAII,6-deoxyerythronolide-B synthase EryA3...
| Macromolecule | Name: EryAII,EryAII,EryAII,EryAII,6-deoxyerythronolide-B synthase EryA3, modules 5 and 6 type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO / EC number: 6-deoxyerythronolide-B synthase |
|---|---|
| Source (natural) | Organism:  Saccharopolyspora erythraea (bacteria) Saccharopolyspora erythraea (bacteria) |
| Molecular weight | Theoretical: 186.489578 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASTDSEKVA EYLRRATLDL RAARQRIREL ESDPIAIVSM ACRLPGGVNT PQRLWELLRE GGETLSGFPT DRGWDLARLH HPDPDNPGT SYVDKGGFLD DAAGFDAEFF GVSPREAAAM DPQQRLLLET SWELVENAGI DPHSLRGTAT GVFLGVAKFG Y GEDTAAAE ...String: MASTDSEKVA EYLRRATLDL RAARQRIREL ESDPIAIVSM ACRLPGGVNT PQRLWELLRE GGETLSGFPT DRGWDLARLH HPDPDNPGT SYVDKGGFLD DAAGFDAEFF GVSPREAAAM DPQQRLLLET SWELVENAGI DPHSLRGTAT GVFLGVAKFG Y GEDTAAAE DVEGYSVTGV APAVASGRIS YTMGLEGPSI SVDTACSSSL VALHLAVESL RKGESSMAVV GGAAVMATPG VF VDFSRQR ALAADGRSKA FGAGADGFGF SEGVTLVLLE RLSEARRNGH EVLAVVRGSA LNQDGASNGL SAPSGPAQRR VIR QALESC GLEPGDVDAV EAHGTGTALG DPIEANALLD TYGRDRDADR PLWLGSVKSN IGHTQAAAGV TGLLKVVLAL RNGE LPATL HVEEPTPHVD WSSGGVALLA GNQPWRRGER TRRAAVSAFG ISGTNAHVIV EEAPEREHRE TTAHDGRPVP LVVSA RTTA ALRAQAAQIA ELLERPDADL AGVGLGLATT RARHEHRAAV VASTREEAVR GLREIAAGAA TADAVVEGVT EVDGRN VVF LFPGQGSQWA GMGAELLSSS PVFAGKIRAC DESMAPMQDW KVSDVLRQAP GAPGLDRVDV VQPVLFAVMV SLAELWR SY GVEPAAVVGH SQGEIAAAHV AGALTLEDAA KLVVGRSRLM RSLSGEGGMA AVALGEAAVR ERLRPWQDRL SVAAVNGP R SVVVSGEPGA LRAFSEDCAA EGIRVRDIDV DYASHSPQIE RVREELLETT GDIAPRPARV TFHSTVESRS MDGTELDAR YWYRNLRETV RFADAVTRLA ESGYDAFIEV SPHPVVVQAV EEAVEEADGA EDAVVVGSLH RDGGDLSAFL RSMATAHVSG VDIRWDVAL PGAAPFALPT YPFQRKRYWL QPAAPAAASD ELAYRVSWTP IEKPESGNLD GDWLVVTPLI SPEWTEMLCE A INANGGRA LRCEVDTSAS RTEMAQAVAQ AGTGFRGVLS LLSSDESACR PGVPAGAVGL LTLVQALGDA GVDAPVWCLT QG AVRTPAD DDLARPAQTT AHGFAQVAGL ELPGRWGGVV DLPESVDDAA LRLLVAVLRG GGRAEDHLAV RDGRLHGRRV VRA SLPQSG SRSWTPHGTV LVTGAASPVG DQLVRWLADR GAERLVLAGA CPGDDLLAAV EEAGASAVVC AQDAAALREA LGDE PVTAL VHAGTLTNFG SISEVAPEEF AETIAAKTAL LAVLDEVLGD RAVEREVYCS SVAGIWGGAG MAAYAAGSAY LDALA EHHR ARGRSCTSVA WTPWALPGGA VDDGYLRERG LRSLSADRAM RTWERVLAAG PVSVAVADVD WPVLSEGFAA TRPTAL FAE LAGRGGQAEA EPDSGPTGEP AQRLAGLSPD EQQENLLELV ANAVAEVLGH ESAAEINVRR AFSELGLD(4HH)L NAM ALRKRL SASTGLRLPA SLVFDHPTVT ALAQHTSQLD SGTPAREASS ALRDGYRQAG VSGRVRSYLD LLAGLSDFRE HFDG SDGFS LDLVDMADGP GEVTVICCAG TAAISGPHEF TRLAGALRGI APVRAVPQPG YEEGEPLPSS MAAVAAVQAD AVIRT QGDK PFVVAGHSAG ALMAYALATE LLDRGHPPRG VVLIDVYPPG HQDAMNAWLE ELTATLFDRE TVRMDDTRLT ALGAYD RLT GQWRPRETGL PTLLVSAGEP MGPWPDDSWK PTWPFEHDTV AVPGDHFTMV QEHADAIARH IDAWLGGGNS SSVDKLA AA LEHHHHHH UniProtKB: 6-deoxyerythronolide-B synthase |
-Macromolecule #2: Antibody Fragment 1B2, Heavy Chain
| Macromolecule | Name: Antibody Fragment 1B2, Heavy Chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 26.447611 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAEVQLVQSG GGLVQPGRSL RLSCTASGFT FGDYAMSWVR QAPGKGLEWV GFIRSKAYGG TTEYAASVKG RFTISRDDSK SIAYLQMNS LKTEDTAVYY CTRGGTLFDY WGQGTLVTVS SASTKGPSVF PLAPSSKSTS GGTAALGCLV KDYFPEPVTV S WNSGALTS ...String: MAEVQLVQSG GGLVQPGRSL RLSCTASGFT FGDYAMSWVR QAPGKGLEWV GFIRSKAYGG TTEYAASVKG RFTISRDDSK SIAYLQMNS LKTEDTAVYY CTRGGTLFDY WGQGTLVTVS SASTKGPSVF PLAPSSKSTS GGTAALGCLV KDYFPEPVTV S WNSGALTS GVHTFPAVLQ SSGLYSLSSV VTVPSSSLGT QTYICNVNHK PSNTKVDKKV EPKSCAALVP RGSAHHHHHH AA DYKDDDD KA |
-Macromolecule #3: Antibody Fragment 1B2, Light Chain
| Macromolecule | Name: Antibody Fragment 1B2, Light Chain / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 25.715832 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: LFAIPLVVPF YSHSALDVVM TQSPLSLPVT PGEPASISCR SSQSLLHSNG YNYLDWYLQK PGQSPQLLIY LGSNRASGVP DRFSGSGSG TDFTLKISRV EAEDVGVYYC MQSLQTPRLT FGPGTKVDIK RTVAAPSVFI FPPSDEQLKS GTASVVCLLN N FYPRGAKV ...String: LFAIPLVVPF YSHSALDVVM TQSPLSLPVT PGEPASISCR SSQSLLHSNG YNYLDWYLQK PGQSPQLLIY LGSNRASGVP DRFSGSGSG TDFTLKISRV EAEDVGVYYC MQSLQTPRLT FGPGTKVDIK RTVAAPSVFI FPPSDEQLKS GTASVVCLLN N FYPRGAKV QWKVDNALQS GNSQESVTEQ DSKDSTYSLS STLTLSKADY EKHKVYACEV THQGLSSPVT KSFNRGEC |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.2 Component:
| ||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 11 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Details: 30 s glow, 10 s hold, 15 mA | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 10480 / Average exposure time: 2.79 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 150.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)