[English] 日本語
 Yorodumi
Yorodumi- EMDB-41458: nhTMEM16 lipid scramblase in lipid nanodiscs with MSP1E3 scaffold... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | nhTMEM16 lipid scramblase in lipid nanodiscs with MSP1E3 scaffold protein in the presence of Ca2+ (closed state)(local refined monomer map) | |||||||||
 Map data Map data | Local refined monomer map used to construct the composite map of nhTMEM16 in lipid nanodiscs with MSP1E3 scaffold protein in the presence of Ca2 (closed state) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | membrane protein / lipid scramblase / TMEM16 / LIPID TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology informationcortical endoplasmic reticulum / chloride channel activity / metal ion binding / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |  Fusarium vanettenii 77-13-4 (fungus) Fusarium vanettenii 77-13-4 (fungus) | |||||||||
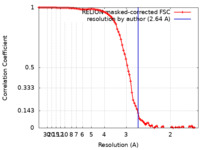
| Method | single particle reconstruction / cryo EM / Resolution: 2.64 Å | |||||||||
 Authors Authors | Feng Z / Accardi A | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Structural basis of closed groove scrambling by a TMEM16 protein. Authors: Zhang Feng / Omar E Alvarenga / Alessio Accardi /  Abstract: Activation of Ca-dependent TMEM16 scramblases induces phosphatidylserine externalization, a key step in multiple signaling processes. Current models suggest that the TMEM16s scramble lipids by ...Activation of Ca-dependent TMEM16 scramblases induces phosphatidylserine externalization, a key step in multiple signaling processes. Current models suggest that the TMEM16s scramble lipids by deforming the membrane near a hydrophilic groove and that Ca dependence arises from the different association of lipids with an open or closed groove. However, the molecular rearrangements underlying groove opening and how lipids reorganize outside the closed groove remain unknown. Here we directly visualize how lipids associate at the closed groove of Ca-bound fungal nhTMEM16 in nanodiscs using cryo-EM. Functional experiments pinpoint lipid-protein interaction sites critical for closed groove scrambling. Structural and functional analyses suggest groove opening entails the sequential appearance of two π-helical turns in the groove-lining TM6 helix and identify critical rearrangements. Finally, we show that the choice of scaffold protein and lipids affects the conformations of nhTMEM16 and their distribution, highlighting a key role of these factors in cryo-EM structure determination. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41458.map.gz emd_41458.map.gz | 12.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41458-v30.xml emd-41458-v30.xml emd-41458.xml emd-41458.xml | 16.4 KB 16.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41458_fsc.xml emd_41458_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_41458.png emd_41458.png | 38.5 KB | ||
| Filedesc metadata |  emd-41458.cif.gz emd-41458.cif.gz | 5.7 KB | ||
| Others |  emd_41458_half_map_1.map.gz emd_41458_half_map_1.map.gz emd_41458_half_map_2.map.gz emd_41458_half_map_2.map.gz | 49.5 MB 49.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41458 http://ftp.pdbj.org/pub/emdb/structures/EMD-41458 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41458 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41458 | HTTPS FTP |
-Related structure data
| Related structure data |  8toiC  8tokC  8tolC  8tpmC  8tpnC  8tpoC  8tppC  8tpqC  8tprC  8tpsC  8tptC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_41458.map.gz / Format: CCP4 / Size: 13.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41458.map.gz / Format: CCP4 / Size: 13.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refined monomer map used to construct the composite map of nhTMEM16 in lipid nanodiscs with MSP1E3 scaffold protein in the presence of Ca2 (closed state) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.825 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: halfmap1
| File | emd_41458_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | halfmap1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: halfmap2
| File | emd_41458_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | halfmap2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Dimeric lipid scramblase nhTMEM16
| Entire | Name: Dimeric lipid scramblase nhTMEM16 |
|---|---|
| Components |
|
-Supramolecule #1: Dimeric lipid scramblase nhTMEM16
| Supramolecule | Name: Dimeric lipid scramblase nhTMEM16 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Fusarium vanettenii 77-13-4 (fungus) Fusarium vanettenii 77-13-4 (fungus) |
-Macromolecule #1: Lipid scramblase nhTMEM16
| Macromolecule | Name: Lipid scramblase nhTMEM16 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Fusarium vanettenii 77-13-4 (fungus) Fusarium vanettenii 77-13-4 (fungus) |
| Sequence | String: GPSNLKDFSQ PGSGQESNFG VDFVIHYKVP AAERDEAEAG FVQLIRALTT VGLATEVRHG ENESLLVFVK VASPDLFAKQ VYRARLGDWL HGVRVSAPHN DIAQALQDEP VVEAERLRLI YLMITKPHNE GGAGVTPTNA KWKHVESIFP LHSHSFNKEW IKKWSSKYTL ...String: GPSNLKDFSQ PGSGQESNFG VDFVIHYKVP AAERDEAEAG FVQLIRALTT VGLATEVRHG ENESLLVFVK VASPDLFAKQ VYRARLGDWL HGVRVSAPHN DIAQALQDEP VVEAERLRLI YLMITKPHNE GGAGVTPTNA KWKHVESIFP LHSHSFNKEW IKKWSSKYTL EQTDIDNIRD KFGESVAFYF AFLRSYFRFL VIPSAFGFGA WLLLGQFSYL YALLCGLWSV VFFEYWKKQE VDLAVQWGVR GVSSIQQSRP EFEWEHEAED PITGEPVKVY PPMKRVKTQL LQIPFALACV VALGALIVTC NSLEVFINEV YSGPGKQYLG FLPTIFLVIG TPTISGVLMG AAEKLNAMEN YATVDAHDAA LIQKQFVLNF MTSYMALFFT AFVYIPFGHI LHPFLNFWRA TAQTLTFSEK ELPTREFQIN PARISNQMFY FTVTAQIVNF ATEVVVPYIK QQAFQKAKQL KSGSKVQEDH EEEAEFLQRV REECTLEEYD VSGDYREMVM QFGYVAMFSV AWPLAACCFL VNNWVELRSD ALKIAISSRR PIPWRTDSIG PWLTALSFLS WLGSITSSAI VYLCSNSKNG TQGEASPLKA WGLLLSILFA EHFYLVVQLA VRFVLSKLDS PGLQKERKER FQTKKRLLQE NLGQDAAEEA AAPGIEHSEK ITREALEEEA RQASIRGHGT PEEMFWQRQR GMQETIEIGR RMIEQQLAAG KNGKKSAPAV PSEKASA UniProtKB: Plasma membrane channel protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Material: GOLD / Support film - Material: GOLD | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 288 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||
| Details | This sample was monodisperse |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 13735 / Average exposure time: 2.0 sec. / Average electron dose: 59.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER / Nominal defocus max: 1.9000000000000001 µm / Nominal defocus min: 0.9 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|
 Movie
Movie Controller
Controller















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)