[English] 日本語
 Yorodumi
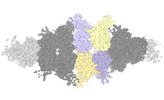
Yorodumi- EMDB-40950: Human liver-type glutaminase (K253A) with L-Gln, filamentous form -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human liver-type glutaminase (K253A) with L-Gln, filamentous form | ||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
 Keywords Keywords | Metabolic / Cancer / HYDROLASE | ||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationL-glutamine catabolic process / glutamate biosynthetic process / Glutamate and glutamine metabolism / glutaminase / Glutamate Neurotransmitter Release Cycle / glutaminase activity / amino acid metabolic process / reactive oxygen species metabolic process / TP53 Regulates Metabolic Genes / regulation of apoptotic process ...L-glutamine catabolic process / glutamate biosynthetic process / Glutamate and glutamine metabolism / glutaminase / Glutamate Neurotransmitter Release Cycle / glutaminase activity / amino acid metabolic process / reactive oxygen species metabolic process / TP53 Regulates Metabolic Genes / regulation of apoptotic process / mitochondrial matrix / mitochondrion Similarity search - Function | ||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.3 Å | ||||||||||||||||||||||||
 Authors Authors | Feng S / Aplin C / Nguyen T-TT / Milano SK / Cerione RA | ||||||||||||||||||||||||
| Funding support |  United States, 7 items United States, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Filament formation drives catalysis by glutaminase enzymes important in cancer progression. Authors: Shi Feng / Cody Aplin / Thuy-Tien T Nguyen / Shawn K Milano / Richard A Cerione /  Abstract: The glutaminase enzymes GAC and GLS2 catalyze the hydrolysis of glutamine to glutamate, satisfying the 'glutamine addiction' of cancer cells. They are the targets of anti-cancer drugs; however, their ...The glutaminase enzymes GAC and GLS2 catalyze the hydrolysis of glutamine to glutamate, satisfying the 'glutamine addiction' of cancer cells. They are the targets of anti-cancer drugs; however, their mechanisms of activation and catalytic activity have been unclear. Here we demonstrate that the ability of GAC and GLS2 to form filaments is directly coupled to their catalytic activity and present their cryo-EM structures which provide a view of the conformational states essential for catalysis. Filament formation guides an 'activation loop' to assume a specific conformation that works together with a 'lid' to close over the active site and position glutamine for nucleophilic attack by an essential serine. Our findings highlight how ankyrin repeats on GLS2 regulate enzymatic activity, while allosteric activators stabilize, and clinically relevant inhibitors block, filament formation that enables glutaminases to catalyze glutaminolysis and support cancer progression. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40950.map.gz emd_40950.map.gz | 32.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40950-v30.xml emd-40950-v30.xml emd-40950.xml emd-40950.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_40950.png emd_40950.png | 68.3 KB | ||
| Filedesc metadata |  emd-40950.cif.gz emd-40950.cif.gz | 5.9 KB | ||
| Others |  emd_40950_half_map_1.map.gz emd_40950_half_map_1.map.gz emd_40950_half_map_2.map.gz emd_40950_half_map_2.map.gz | 200.5 MB 200.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40950 http://ftp.pdbj.org/pub/emdb/structures/EMD-40950 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40950 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40950 | HTTPS FTP |
-Validation report
| Summary document |  emd_40950_validation.pdf.gz emd_40950_validation.pdf.gz | 994.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_40950_full_validation.pdf.gz emd_40950_full_validation.pdf.gz | 994.4 KB | Display | |
| Data in XML |  emd_40950_validation.xml.gz emd_40950_validation.xml.gz | 14 KB | Display | |
| Data in CIF |  emd_40950_validation.cif.gz emd_40950_validation.cif.gz | 15.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40950 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40950 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40950 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40950 | HTTPS FTP |
-Related structure data
| Related structure data |  8t0zMC  8szjC  8szlC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40950.map.gz / Format: CCP4 / Size: 34.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40950.map.gz / Format: CCP4 / Size: 34.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.058 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_40950_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
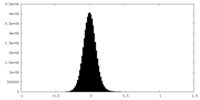
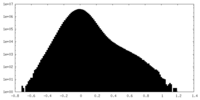
| Density Histograms |
-Half map: #1
| File | emd_40950_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
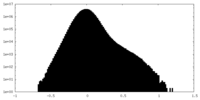
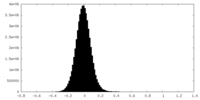
| Density Histograms |
- Sample components
Sample components
-Entire : Human liver-type glutaminase (K253A) with L-Gln, filamentous form
| Entire | Name: Human liver-type glutaminase (K253A) with L-Gln, filamentous form |
|---|---|
| Components |
|
-Supramolecule #1: Human liver-type glutaminase (K253A) with L-Gln, filamentous form
| Supramolecule | Name: Human liver-type glutaminase (K253A) with L-Gln, filamentous form type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Glutaminase liver isoform, mitochondrial
| Macromolecule | Name: Glutaminase liver isoform, mitochondrial / type: protein_or_peptide / ID: 1 / Number of copies: 12 / Enantiomer: LEVO / EC number: glutaminase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 66.351617 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRSMKALQKA LSRAGSHCGR GGWGHPSRSP LLGGGVRHHL SEAAAQGRET PHSHQPQHQD HDSSESGMLS RLGDLLFYTI AEGQERIPI HKFTTALKAT GLQTSDPRLR DCMSEMHRVV QESSSGGLLD RDLFRKCVSS NIVLLTQAFR KKFVIPDFEE F TGHVDRIF ...String: MRSMKALQKA LSRAGSHCGR GGWGHPSRSP LLGGGVRHHL SEAAAQGRET PHSHQPQHQD HDSSESGMLS RLGDLLFYTI AEGQERIPI HKFTTALKAT GLQTSDPRLR DCMSEMHRVV QESSSGGLLD RDLFRKCVSS NIVLLTQAFR KKFVIPDFEE F TGHVDRIF EDVKELTGGK VAAYIPQLAK SNPDLWGVSL CTVDGQRHSV GHTKIPFCLQ SCVKPLTYAI SISTLGTDYV HK FVGKEPS GLRYNALSLN EEGIPHNPMV NAGAIVVSSL IKMDCNKAEK FDFVLQYLNK MAGNEYMGFS NATFQSEKET GDR NYAIGY YLKEKKCFPK GVDMMAALDL YFQLCSVEVT CESGSVMAAT LANGGICPIT GESVLSAEAV RNTLSLMHSC GMYD FSGQF AFHVGLPAKS AVSGAILLVV PNVMGMMCLS PPLDKLGNSH RGTSFCQKLV SLFNFHNYDN LRHCARKLDP RREGA EIRN KTVVNLLFAA YSGDVSALRR FALSAMDMEQ KDYDSRTALH VAAAEGHIEV VKFLIEACKV NPFAKDRWGN IPLDDA VQF NHLEVVKLLQ DYQDSYTLSE TQAEAAAEAL SKENLESMV UniProtKB: Glutaminase liver isoform, mitochondrial |
-Macromolecule #2: GLUTAMINE
| Macromolecule | Name: GLUTAMINE / type: ligand / ID: 2 / Number of copies: 12 / Formula: GLN |
|---|---|
| Molecular weight | Theoretical: 146.144 Da |
| Chemical component information |  ChemComp-GLN: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 66.0 Å Applied symmetry - Helical parameters - Δ&Phi: 48 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 48000 |
|---|---|
| Startup model | Type of model: NONE |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller






 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)