[English] 日本語
 Yorodumi
Yorodumi- EMDB-40930: Reconstituted E. coli RNA polymerase post-termination complex on ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Reconstituted E. coli RNA polymerase post-termination complex on negatively-supercoiled DNA: closed duplex DNA (rPTCc) | |||||||||
 Map data Map data | Locally Filtered | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA polymerase / Negatively supercoiled DNA / Sigma-independent transcription / TRANSCRIPTION | |||||||||
| Function / homology |  Function and homology information Function and homology informationDNA-directed RNA polymerase complex / ribonucleoside binding / DNA-directed RNA polymerase / DNA-directed RNA polymerase activity / protein dimerization activity / DNA-templated transcription / magnesium ion binding / DNA binding / zinc ion binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.69 Å | |||||||||
 Authors Authors | Brewer JJ / Darst SA / Campbell EA | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2025 Journal: Nat Struct Mol Biol / Year: 2025Title: RapA opens the RNA polymerase clamp to disrupt post-termination complexes and prevent cytotoxic R-loop formation. Authors: Joshua J Brewer / Koe Inlow / Rachel A Mooney / Barbara Bosch / Paul Dominic B Olinares / Leandro Pimentel Marcelino / Brian T Chait / Robert Landick / Jeff Gelles / Elizabeth A Campbell / Seth A Darst /  Abstract: Following transcript release during intrinsic termination, Escherichia coli RNA polymerase (RNAP) often remains associated with DNA in a post-termination complex (PTC). RNAPs in PTCs are removed from ...Following transcript release during intrinsic termination, Escherichia coli RNA polymerase (RNAP) often remains associated with DNA in a post-termination complex (PTC). RNAPs in PTCs are removed from the DNA by the SWI2/SNF2 adenosine triphosphatase (ATPase) RapA. Here we determined PTC structures on negatively supercoiled DNA and with RapA engaged to dislodge the PTC. We found that core RNAP in the PTC can unwind DNA and initiate RNA synthesis but is prone to producing R-loops. Nucleotide binding to RapA triggers a conformational change that opens the RNAP clamp, allowing DNA in the RNAP cleft to reanneal and dissociate. We show that RapA helps to control cytotoxic R-loop formation in vivo, likely by disrupting PTCs. We suggest that analogous ATPases acting on PTCs to suppress transcriptional noise and R-loop formation may be widespread. These results hold importance for the bacterial transcription cycle and highlight a role for RapA in maintaining genome stability. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40930.map.gz emd_40930.map.gz | 3.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40930-v30.xml emd-40930-v30.xml emd-40930.xml emd-40930.xml | 25.5 KB 25.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_40930.png emd_40930.png | 71.5 KB | ||
| Filedesc metadata |  emd-40930.cif.gz emd-40930.cif.gz | 8.4 KB | ||
| Others |  emd_40930_additional_1.map.gz emd_40930_additional_1.map.gz emd_40930_half_map_1.map.gz emd_40930_half_map_1.map.gz emd_40930_half_map_2.map.gz emd_40930_half_map_2.map.gz | 59.4 MB 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40930 http://ftp.pdbj.org/pub/emdb/structures/EMD-40930 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40930 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40930 | HTTPS FTP |
-Related structure data
| Related structure data |  8t00MC  8szwC  8t02C  8t0lC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40930.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40930.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally Filtered | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.076 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Sharpened
| File | emd_40930_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
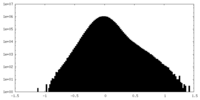
| Density Histograms |
-Half map: Half A
| File | emd_40930_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half A | ||||||||||||
| Projections & Slices |
| ||||||||||||
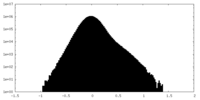
| Density Histograms |
-Half map: Half B
| File | emd_40930_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : E. coli Post-termination complex with open DNA bubble on negative...
| Entire | Name: E. coli Post-termination complex with open DNA bubble on negatively supercoiled DNA |
|---|---|
| Components |
|
-Supramolecule #1: E. coli Post-termination complex with open DNA bubble on negative...
| Supramolecule | Name: E. coli Post-termination complex with open DNA bubble on negatively supercoiled DNA type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2, #4-#5 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: DNA-directed RNA polymerase subunit alpha
| Macromolecule | Name: DNA-directed RNA polymerase subunit alpha / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: DNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 25.897453 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SVTEFLKPRL VDIEQVSSTH AKVTLEPLER GFGHTLGNAL RRILLSSMPG CAVTEVEIDG VLHEYSTKEG VQEDILEILL NLKGLAVRV QGKDEVILTL NKSGIGPVTA ADITHDGDVE IVKPQHVICH LTDENASISM RIKVQRGRGY VPASTRIHSE E DERPIGRL ...String: SVTEFLKPRL VDIEQVSSTH AKVTLEPLER GFGHTLGNAL RRILLSSMPG CAVTEVEIDG VLHEYSTKEG VQEDILEILL NLKGLAVRV QGKDEVILTL NKSGIGPVTA ADITHDGDVE IVKPQHVICH LTDENASISM RIKVQRGRGY VPASTRIHSE E DERPIGRL LVDACYSPVE RIAYNVEAAR VEQRTDLDKL VIEMETNGTI DPEEAIRRAA TILAEQLEAF VDLRDV UniProtKB: DNA-directed RNA polymerase subunit alpha |
-Macromolecule #2: DNA-directed RNA polymerase subunit beta
| Macromolecule | Name: DNA-directed RNA polymerase subunit beta / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 150.560562 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: VYSYTEKKRI RKDFGKRPQV LDVPYLLSIQ LDSFQKFIEQ DPEGQYGLEA AFRSVFPIQS YSGNSELQYV SYRLGEPVFD VQECQIRGV TYSAPLRVKL RLVIYEREAP EGTVKDIKEQ EVYMGEIPLM TDNGTFVING TERVIVSQLH RSPGVFFDSD K GKTHSSGK ...String: VYSYTEKKRI RKDFGKRPQV LDVPYLLSIQ LDSFQKFIEQ DPEGQYGLEA AFRSVFPIQS YSGNSELQYV SYRLGEPVFD VQECQIRGV TYSAPLRVKL RLVIYEREAP EGTVKDIKEQ EVYMGEIPLM TDNGTFVING TERVIVSQLH RSPGVFFDSD K GKTHSSGK VLYNARIIPY RGSWLDFEFD PKDNLFVRID RRRKLPATII LRALNYTTEQ ILDLFFEKVI FEIRDNKLQM EL VPERLRG ETASFDIEAN GKVYVEKGRR ITARHIRQLE KDDVKLIEVP VEYIAGKVVA KDYIDESTGE LICAANMELS LDL LAKLSQ SGHKRIETLF TNDLDHGPYI SETLRVDPTN DRLSALVEIY RMMRPGEPPT REAAESLFEN LFFSEDRYDL SAVG RMKFN RSLLREEIEG SGILSKDDII DVMKKLIDIR NGKGEVDDID HLGNRRIRSV GEMAENQFRV GLVRVERAVK ERLSL GDLD TLMPQDMINA KPISAAVKEF FGSSQLSQFM DQNNPLSEIT HKRRISALGP GGLTRERAGF EVRDVHPTHY GRVCPI ETP EGPNIGLINS LSVYAQTNEY GFLETPYRKV TDGVVTDEIH YLSAIEEGNY VIAQANSNLD EEGHFVEDLV TCRSKGE SS LFSRDQVDYM DVSTQQVVSV GASLIPFLEH DDANRALMGA NMQRQAVPTL RADKPLVGTG MERAVAVDSG VTAVAKRG G VVQYVDASRI VIKVNEDEMY PGEAGIDIYN LTKYTRSNQN TCINQMPCVS LGEPVERGDV LADGPSTDLG ELALGQNMR VAFMPWNGYN FEDSILVSER VVQEDRFTTI HIQELACVSR DTKLGPEEIT ADIPNVGEAA LSKLDESGIV YIGAEVTGGD ILVGKVTPK GETQLTPEEK LLRAIFGEKA SDVKDSSLRV PNGVSGTVID VQVFTRDGVE KDKRALEIEE MQLKQAKKDL S EELQILEA GLFSRIRAVL VAGGVEAEKL DKLPRDRWLE LGLTDEEKQN QLEQLAEQYD ELKHEFEKKL EAKRRKITQG DD LAPGVLK IVKVYLAVKR RIQPGDKMAG RHGNKGVISK INPIEDMPYD ENGTPVDIVL NPLGVPSRMN IGQILETHLG MAA KGIGDK INAMLKQQQE VAKLREFIQR AYDLGADVRQ KVDLSTFSDE EVMRLAENLR KGMPIATPVF DGAKEAEIKE LLKL GDLPT SGQIRLYDGR TGEQFERPVT VGYMYMLKLN HLVDDKMHAR STGSYSLVTQ QPLGGKAQFG GQRFGEMEVW ALEAY GAAY TLQEMLTVKS DDVNGRTKMY KNIVDGNHQM EPGMPESFNV LLKEIRSLGI NIELED UniProtKB: DNA-directed RNA polymerase subunit beta |
-Macromolecule #3: DNA-directed RNA polymerase subunit beta'
| Macromolecule | Name: DNA-directed RNA polymerase subunit beta' / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: DNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 150.350234 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: EFDAIKIALA SPDMIRSWSF GEVKKPETIN YRTFKPERDG LFCARIFGPV KDYECLCGKY KRLKHRGVIC EKCGVEVTQT KVRRERMGH IELASPTAHI WFLKSLPSRI GLLLDMPLRD IERVLYFESY VVIEGGMTNL ERQQILTEEQ YLDALEEFGD E FDAKMGAE ...String: EFDAIKIALA SPDMIRSWSF GEVKKPETIN YRTFKPERDG LFCARIFGPV KDYECLCGKY KRLKHRGVIC EKCGVEVTQT KVRRERMGH IELASPTAHI WFLKSLPSRI GLLLDMPLRD IERVLYFESY VVIEGGMTNL ERQQILTEEQ YLDALEEFGD E FDAKMGAE AIQALLKSMD LEQECEQLRE ELNETNSETK RKKLTKRIKL LEAFVQSGNK PEWMILTVLP VLPPDLRPLV PL DGGRFAT SDLNDLYRRV INRNNRLKRL LDLAAPDIIV RNEKRMLQEA VDALLDNGRR GRAITGSNKR PLKSLADMIK GKQ GRFRQN LLGKRVDYSG RSVITVGPYL RLHQCGLPKK MALELFKPFI YGKLELRGLA TTIKAAKKMV EREEAVVWDI LDEV IREHP VLLNRAPTLH RLGIQAFEPV LIEGKAIQLH PLVCAAYNAD FDGDQMAVHV PLTLEAQLEA RALMMSTNNI LSPAN GEPI IVPSQDVVLG LYYMTRDCVN AKGEGMVLTG PKEAERLYRS GLASLHARVK VRITEYEKDA NGELVAKTSL KDTTVG RAI LWMIVPKGLP YSIVNQALGK KAISKMLNTC YRILGLKPTV IFADQIMYTG FAYAARSGAS VGIDDMVIPE KKHEIIS EA EAEVAEIQEQ FQSGLVTAGE RYNKVIDIWA AANDRVSKAM MDNLQTETVI NRDGQEEKQV SFNSIYMMAD SGARGSAA Q IRQLAGMRGL MAKPDGSIIE TPITANFREG LNVLQYFIST HGARKGLADT ALKTANSGYL TRRLVDVAQD LVVTEDDCG THEGIMMTPV IEGGDVKEPL RDRVLGRVTA EDVLKPGTAD ILVPRNTLLH EQWCDLLEEN SVDAVKVRSV VSCDTDFGVC AHCYGRDLA RGHIINKGEA IGVIAAQSIG EPGTQLTMRT FHIGGAASRA AAESSIQVKN KGSIKLSNVK SVVNSSGKLV I TSRNTELK LIDEFGRTKE SYKVPYGAVL AKGDGEQVAG GETVANWDPH TMPVITEVSG FVRFTDMIDG QTITRQTDEL TG LSSLVVL DSAERTAGGK DLRPALKIVD AQGNDVLIPG TDMPAQYFLP GKAIVQLEDG VQISSGDTLA RIPQESGGTK DIT GGLPRV ADLFEARRPK EPAILAEISG IVSFGKETKG KRRLVITPVD GSDPYEEMIP KWRQLNVFEG ERVERGDVIS DGPE APHDI LRLRGVHAVT RYIVNEVQDV YRLQGVKIND KHIEVIVRQM LRKATIVNAG SSDFLEGEQV EYSRVKIANR ELEAN GKVG ATYSRDLLGI TKASLATESF ISAASFQETT RVLTEAAVAG KRDELRGLKE NVIVGRLIPA GTGYAYHQDA MRRR UniProtKB: DNA-directed RNA polymerase subunit beta' |
-Macromolecule #4: DNA (26-MER)
| Macromolecule | Name: DNA (26-MER) / type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 8.098421 KDa |
| Sequence | String: (DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA) (DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA) (DA)(DA)(DA)(DA)(DA)(DA) |
-Macromolecule #5: DNA (26-MER)
| Macromolecule | Name: DNA (26-MER) / type: dna / ID: 5 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 7.864056 KDa |
| Sequence | String: (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT) |
-Macromolecule #6: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 6 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #7: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 7 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 55.9 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)