[English] 日本語
 Yorodumi
Yorodumi- EMDB-3936: In situ subtomogram average of the Chlamydomonas membrane-tethere... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3936 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | In situ subtomogram average of the Chlamydomonas membrane-tethered 26S proteasome | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 24.87 Å | |||||||||
 Authors Authors | Albert S / Schaffer M / Beck F / Mosalaganti S / Asano S / Thomas HF / Plitzko J / Beck M / Baumeister W / Engel BD | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2017 Journal: Proc Natl Acad Sci U S A / Year: 2017Title: Proteasomes tether to two distinct sites at the nuclear pore complex. Authors: Sahradha Albert / Miroslava Schaffer / Florian Beck / Shyamal Mosalaganti / Shoh Asano / Henry F Thomas / Jürgen M Plitzko / Martin Beck / Wolfgang Baumeister / Benjamin D Engel /  Abstract: The partitioning of cellular components between the nucleus and cytoplasm is the defining feature of eukaryotic life. The nuclear pore complex (NPC) selectively gates the transport of macromolecules ...The partitioning of cellular components between the nucleus and cytoplasm is the defining feature of eukaryotic life. The nuclear pore complex (NPC) selectively gates the transport of macromolecules between these compartments, but it is unknown whether surveillance mechanisms exist to reinforce this function. By leveraging in situ cryo-electron tomography to image the native cellular environment of , we observed that nuclear 26S proteasomes crowd around NPCs. Through a combination of subtomogram averaging and nanometer-precision localization, we identified two classes of proteasomes tethered via their Rpn9 subunits to two specific NPC locations: binding sites on the NPC basket that reflect its eightfold symmetry and more abundant binding sites at the inner nuclear membrane that encircle the NPC. These basket-tethered and membrane-tethered proteasomes, which have similar substrate-processing state frequencies as proteasomes elsewhere in the cell, are ideally positioned to regulate transcription and perform quality control of both soluble and membrane proteins transiting the NPC. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3936.map.gz emd_3936.map.gz | 10.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3936-v30.xml emd-3936-v30.xml emd-3936.xml emd-3936.xml | 12.6 KB 12.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_3936.png emd_3936.png | 30.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3936 http://ftp.pdbj.org/pub/emdb/structures/EMD-3936 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3936 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3936 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_3936.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3936.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.42 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
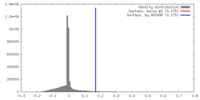
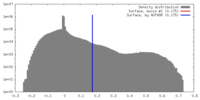
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : In situ membrane-tethered 26S proteasome
| Entire | Name: In situ membrane-tethered 26S proteasome |
|---|---|
| Components |
|
-Supramolecule #1: In situ membrane-tethered 26S proteasome
| Supramolecule | Name: In situ membrane-tethered 26S proteasome / type: complex / ID: 1 / Parent: 0 Details: In situ subtomogram average generated from membrane-tethered 26S proteasomes imaged within the native Chlamydomonas cell. Cells were thinned by focused ion beam milling. |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 2 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 90 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV Details: Blotted for 10 seconds with 10 blot force before plunging.. |
| Details | Membrane-tethered 26S proteasome within the nucleus of the native Chlamydomonas cell. Whole cells were plunge-frozen onto EM grids and then thinned with a cryo-focused ion beam instrument. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Lower energy threshold: 0 eV / Energy filter - Upper energy threshold: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Average exposure time: 1.5 sec. / Average electron dose: 1.5 e/Å2 Details: Images were collected in movie mode at 12 frames per second |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 6.0 µm / Nominal defocus min: 4.0 µm / Nominal magnification: 42000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)