[English] 日本語
 Yorodumi
Yorodumi- EMDB-38355: Structure of the Argonaute protein from Kurthia massiliensis in c... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the Argonaute protein from Kurthia massiliensis in complex with guide DNA | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Mesophilic prokaryotic Argonaute / DNA BINDING PROTEIN-DNA COMPLEX | |||||||||
| Biological species |  Kurthia massiliensis (bacteria) Kurthia massiliensis (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.81 Å | |||||||||
 Authors Authors | Xin T / Hui D / Shaowen W | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2024 Journal: Nucleic Acids Res / Year: 2024Title: Structural and mechanistic insights into a mesophilic prokaryotic Argonaute. Authors: Xin Tao / Hui Ding / Shaowen Wu / Fei Wang / Hu Xu / Jie Li / Chao Zhai / Shunshun Li / Kai Chen / Shan Wu / Yang Liu / Lixin Ma /  Abstract: Argonaute (Ago) proteins are programmable nucleases found in all domains of life, playing a crucial role in biological processes like DNA/RNA interference and gene regulation. Mesophilic prokaryotic ...Argonaute (Ago) proteins are programmable nucleases found in all domains of life, playing a crucial role in biological processes like DNA/RNA interference and gene regulation. Mesophilic prokaryotic Agos (pAgos) have gained increasing research interest due to their broad range of potential applications, yet their molecular mechanisms remain poorly understood. Here, we present seven cryo-electron microscopy structures of Kurthia massiliensis Ago (KmAgo) in various states. These structures encompass the steps of apo-form, guide binding, target recognition, cleavage, and release, revealing that KmAgo employs a unique DDD catalytic triad, instead of a DEDD tetrad, for DNA target cleavage under 5'P-DNA guide conditions. Notably, the last catalytic residue, D713, is positioned outside the catalytic pocket in the absence of guide. After guide binding, D713 enters the catalytic pocket. In contrast, the corresponding catalytic residue in other Agos has been consistently located in the catalytic pocket. Moreover, we identified several sites exhibiting enhanced catalytic activity through alanine mutagenesis. These sites have the potential to serve as engineering targets for augmenting the catalytic efficiency of KmAgo. This structural analysis of KmAgo advances the understanding of the diversity of molecular mechanisms by Agos, offering insights for developing and optimizing mesophilic pAgos-based programmable DNA and RNA manipulation tools. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38355.map.gz emd_38355.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38355-v30.xml emd-38355-v30.xml emd-38355.xml emd-38355.xml | 23.5 KB 23.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_38355_fsc.xml emd_38355_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_38355.png emd_38355.png | 162.1 KB | ||
| Filedesc metadata |  emd-38355.cif.gz emd-38355.cif.gz | 7.3 KB | ||
| Others |  emd_38355_half_map_1.map.gz emd_38355_half_map_1.map.gz emd_38355_half_map_2.map.gz emd_38355_half_map_2.map.gz | 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38355 http://ftp.pdbj.org/pub/emdb/structures/EMD-38355 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38355 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38355 | HTTPS FTP |
-Related structure data
| Related structure data |  8xhvMC  8xhsC  8xjwC  8xjxC  8xk0C  8xk3C  8xk4C M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_38355.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38355.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.851 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_38355_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
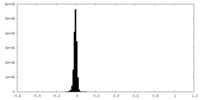
| Density Histograms |
-Half map: #1
| File | emd_38355_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
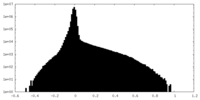
| Density Histograms |
- Sample components
Sample components
-Entire : Structure of the Argonaute protein from Kurthia massiliensis in c...
| Entire | Name: Structure of the Argonaute protein from Kurthia massiliensis in complex with guide DNA |
|---|---|
| Components |
|
-Supramolecule #1: Structure of the Argonaute protein from Kurthia massiliensis in c...
| Supramolecule | Name: Structure of the Argonaute protein from Kurthia massiliensis in complex with guide DNA type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Kurthia massiliensis (bacteria) Kurthia massiliensis (bacteria) |
-Macromolecule #1: KmAgo
| Macromolecule | Name: KmAgo / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Kurthia massiliensis (bacteria) Kurthia massiliensis (bacteria) |
| Molecular weight | Theoretical: 85.485203 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MEAYITEMVS RERANELEVY VYVFPRKQSD NNYEGVYHIM RAWQRANDLP LAYNQHTIMA FSPVRHMCGY TPMETQKRHI NIDSPFERA LLERLIKNSL IFTAERHLHA KRVGHALRLN QVQQIRQVII YEAIELYVNI IENRISIGFH LTHQFEYVYT L QSMIEQGK ...String: MEAYITEMVS RERANELEVY VYVFPRKQSD NNYEGVYHIM RAWQRANDLP LAYNQHTIMA FSPVRHMCGY TPMETQKRHI NIDSPFERA LLERLIKNSL IFTAERHLHA KRVGHALRLN QVQQIRQVII YEAIELYVNI IENRISIGFH LTHQFEYVYT L QSMIEQGK TIRPGMRVVH SNGRQHYTYT VENVATYGVT DRCPLLQTSI YQYYVEKGAQ HILRTFTRST RVIHVRTKEQ RL SYAATLL KPLCTFETMQ PQDVLNVSKC IKLSASKRMK CTYRWIQQLR AQYRHLTFAP NPFTIAQNGY KLDQLSTPKV HFH RDYATV VSGMKTGKLY KGGNIKISVL FDEDFYLKHH ITKKDIYQFI AVLQKIAIAQ GVNMTISTST KSITGKFTDD FFHH FTEEV EALQPIFAQT TVLAFITSTH LSNKKTRSYQ LLKQYFGGKW DIASQVITEK TIEAFQKILH KHGLKNFYPN DEQHC LRVI DVLKNESFYY TVMNILLGVY VKSGIQPWIL ANTTHSDCFI GIDVSHENGN SAAGMMNVIG SQGHLIQQAP LNGILA GEK IDDTLLANLL KQMIKAYHTQ FQRFPKHITI HRDGFWREHT ALVEKIMSHY EITYDIVEII KKPNRRMAFF NSVDNTF ST RQGTVYQRGN EAFLCATNPQ QKVGMAQPIK IHQVTKTLPF SHIIEDVYNL SFLHIHAMNK MRLPATIHYA DLSATAYQ R GQVMPRSGNQ TNLPFV |
-Macromolecule #2: guide DNA
| Macromolecule | Name: guide DNA / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Kurthia massiliensis (bacteria) Kurthia massiliensis (bacteria) |
| Molecular weight | Theoretical: 5.64166 KDa |
| Sequence | String: (DT)(DG)(DA)(DG)(DG)(DT)(DA)(DG)(DT)(DA) (DG)(DG)(DT)(DT)(DG)(DT)(DA)(DT) |
-Macromolecule #3: MANGANESE (II) ION
| Macromolecule | Name: MANGANESE (II) ION / type: ligand / ID: 3 / Number of copies: 1 / Formula: MN |
|---|---|
| Molecular weight | Theoretical: 54.938 Da |
-Macromolecule #4: water
| Macromolecule | Name: water / type: ligand / ID: 4 / Number of copies: 24 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 54.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)