[English] 日本語
 Yorodumi
Yorodumi- EMDB-36865: Spiral pentameric form of the substrate-free Lon protease with a ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Spiral pentameric form of the substrate-free Lon protease with a Y224S mutation in the presence of the N-terminal-truncated monomeric mutant bearing an E613K mutation | |||||||||
 Map data Map data | spiral pentamer of Y224S:dN-E613K:ADP | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | AAA+ protein / ATPase / HYDROLASE | |||||||||
| Biological species |  Meiothermus taiwanensis (bacteria) Meiothermus taiwanensis (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Li S / Hsieh KY / Kuo CI / Zhang K / Chang CI | |||||||||
| Funding support |  Taiwan, 2 items Taiwan, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: A 5+1 assemble-to-activate mechanism of the Lon proteolytic machine. Authors: Shanshan Li / Kan-Yen Hsieh / Chiao-I Kuo / Tzu-Chi Lin / Szu-Hui Lee / Yi-Ru Chen / Chun-Hsiung Wang / Meng-Ru Ho / See-Yeun Ting / Kaiming Zhang / Chung-I Chang /   Abstract: Many AAA+ (ATPases associated with diverse cellular activities) proteins function as protein or DNA remodelers by threading the substrate through the central pore of their hexameric assemblies. In ...Many AAA+ (ATPases associated with diverse cellular activities) proteins function as protein or DNA remodelers by threading the substrate through the central pore of their hexameric assemblies. In this ATP-dependent translocating state, the substrate is gripped by the pore loops of the ATPase domains arranged in a universal right-handed spiral staircase organization. However, the process by which a AAA+ protein is activated to adopt this substrate-pore-loop arrangement remains unknown. We show here, using cryo-electron microscopy (cryo-EM), that the activation process of the Lon AAA+ protease may involve a pentameric assembly and a substrate-dependent incorporation of the sixth protomer to form the substrate-pore-loop contacts seen in the translocating state. Based on the structural results, we design truncated monomeric mutants that inhibit Lon activity by binding to the native pentamer and demonstrated that expressing these monomeric mutants in Escherichia coli cells containing functional Lon elicits specific phenotypes associated with lon deficiency, including the inhibition of persister cell formation. These findings uncover a substrate-dependent assembly process for the activation of a AAA+ protein and demonstrate a targeted approach to selectively inhibit its function within cells. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36865.map.gz emd_36865.map.gz | 72.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36865-v30.xml emd-36865-v30.xml emd-36865.xml emd-36865.xml | 18.1 KB 18.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36865_fsc.xml emd_36865_fsc.xml | 11.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_36865.png emd_36865.png | 65.9 KB | ||
| Filedesc metadata |  emd-36865.cif.gz emd-36865.cif.gz | 5.5 KB | ||
| Others |  emd_36865_additional_1.map.gz emd_36865_additional_1.map.gz emd_36865_half_map_1.map.gz emd_36865_half_map_1.map.gz emd_36865_half_map_2.map.gz emd_36865_half_map_2.map.gz | 136.8 MB 134.4 MB 134.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36865 http://ftp.pdbj.org/pub/emdb/structures/EMD-36865 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36865 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36865 | HTTPS FTP |
-Related structure data
| Related structure data |  7yphC  7ypiC  7ypjC  7ypkC  7yuhC  7yumC  7yupC  7yutC  7yuuC  7yuvC  7yuwC  7yuxC  8k3yC C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_36865.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36865.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | spiral pentamer of Y224S:dN-E613K:ADP | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.061 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: sharpened map
| File | emd_36865_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
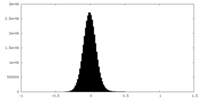
| Density Histograms |
-Half map: half map B
| File | emd_36865_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
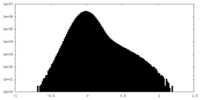
| Density Histograms |
-Half map: half map A
| File | emd_36865_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Spiral pentamer of the substrate-free Lon protease with a Y224S m...
| Entire | Name: Spiral pentamer of the substrate-free Lon protease with a Y224S mutation in the presence of the N-terminal-truncated monomeric mutant bearing a E613K mutation. |
|---|---|
| Components |
|
-Supramolecule #1: Spiral pentamer of the substrate-free Lon protease with a Y224S m...
| Supramolecule | Name: Spiral pentamer of the substrate-free Lon protease with a Y224S mutation in the presence of the N-terminal-truncated monomeric mutant bearing a E613K mutation. type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Meiothermus taiwanensis (bacteria) Meiothermus taiwanensis (bacteria) |
-Macromolecule #1: homo-pentamer of Lon protease with a Y224S mutation
| Macromolecule | Name: homo-pentamer of Lon protease with a Y224S mutation / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Meiothermus taiwanensis (bacteria) Meiothermus taiwanensis (bacteria) |
| Recombinant expression | Organism:  |
| Sequence | String: MRLELPVIPL RNTVILPHTT TPVDVGRAKS KRAVEEAMGA DRLIFLVAQR DPEVDDPAPD DLYTWGVQAV VKQAMRLPDG TLQVMVEARA RAQVTDYIPG PYLRARGEVF SEIFPIDEAV VRVLVEELKE AFEKYVANHK SLRLDRYQLE AVKGTSDPAM LADTIAYHAT ...String: MRLELPVIPL RNTVILPHTT TPVDVGRAKS KRAVEEAMGA DRLIFLVAQR DPEVDDPAPD DLYTWGVQAV VKQAMRLPDG TLQVMVEARA RAQVTDYIPG PYLRARGEVF SEIFPIDEAV VRVLVEELKE AFEKYVANHK SLRLDRYQLE AVKGTSDPAM LADTIAYHAT WTVAEKQEIL ELTDLEARLK KVLGLLSRDL ERFELDKRVA QRVKEQMDTN QRESYLREQM KAIQKELGGE DGLSDLEALR KKIEEVGMPE AVKTKALKEL DRLERMQQGS PEATVARTYL DWLTEVPWSK ADPEVLDINH TRQVLDEDHY GLKDVKERIL EYLAVRQLTQ GLDVRNKAPI LVLVGPPGVG KTSLGRSIAR SMNRKFHRIS LGGVRDEAEI RGHRRTYIGA MPGKLIHAMK QVGVINPVIL LDEIDKMSSD WRGDPASAML EVLDPEQNNT FTDHYLDVPY DLSKVFFITT ANTLQTIPRP LLDRMEVIEI PGYTNMEKQA IARQYLWPKQ VRESGMEGRI EVTDAAILRV ISEYTREAGV RGLERELGKI ARKGAKFWLE GAWEGLRTID ASDIPTYLGI PRYRPDKAET EPQVGTAQGL AWTPVGGTLL TIEVAAVPGS GKLSLTGQLG EVMKESAQAA LTYLRAHTQD YGLPEDFYNK VDLHVHVPDG ATPKDGPSAG ITMATAIASA LSRRPARMDI AMTGEVSLRG KVMPIGGVKE KLLAAHQAGI HKIVLPKDNE AQLEELPKEV LEGLEIKLVE DVGEVLEYLL LPEPTMPPVV QPSDNRQQPG AGAHHHHHH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.7 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: 20 mM Tris-HCl, 200 mM NaCl, 10 mM MgCl2, 1 mM DTT | |||||||||||||||
| Grid | Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. | |||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.4000000000000001 µm / Nominal magnification: 81000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)