[English] 日本語
 Yorodumi
Yorodumi- EMDB-34000: Open-spiral pentamer of the substrate-free Lon protease with a Y2... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Open-spiral pentamer of the substrate-free Lon protease with a Y224S mutation | |||||||||
 Map data Map data | Pentameric state of MtaLon-Y224S with ATPgammaS | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Lon protease / hydrolysis / AAA proteins / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationendopeptidase La / ATP-dependent peptidase activity / protein quality control for misfolded or incompletely synthesized proteins / cellular response to heat / sequence-specific DNA binding / serine-type endopeptidase activity / ATP hydrolysis activity / ATP binding / metal ion binding / identical protein binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Meiothermus taiwanensis (bacteria) Meiothermus taiwanensis (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.68 Å | |||||||||
 Authors Authors | Li S / Hsieh KY / Kuo CI / Lee SH / Ho MR / Wang CH / Zhang K / Chang CI | |||||||||
| Funding support |  Taiwan, 1 items Taiwan, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: A 5+1 assemble-to-activate mechanism of the Lon proteolytic machine. Authors: Shanshan Li / Kan-Yen Hsieh / Chiao-I Kuo / Tzu-Chi Lin / Szu-Hui Lee / Yi-Ru Chen / Chun-Hsiung Wang / Meng-Ru Ho / See-Yeun Ting / Kaiming Zhang / Chung-I Chang /   Abstract: Many AAA+ (ATPases associated with diverse cellular activities) proteins function as protein or DNA remodelers by threading the substrate through the central pore of their hexameric assemblies. In ...Many AAA+ (ATPases associated with diverse cellular activities) proteins function as protein or DNA remodelers by threading the substrate through the central pore of their hexameric assemblies. In this ATP-dependent translocating state, the substrate is gripped by the pore loops of the ATPase domains arranged in a universal right-handed spiral staircase organization. However, the process by which a AAA+ protein is activated to adopt this substrate-pore-loop arrangement remains unknown. We show here, using cryo-electron microscopy (cryo-EM), that the activation process of the Lon AAA+ protease may involve a pentameric assembly and a substrate-dependent incorporation of the sixth protomer to form the substrate-pore-loop contacts seen in the translocating state. Based on the structural results, we design truncated monomeric mutants that inhibit Lon activity by binding to the native pentamer and demonstrated that expressing these monomeric mutants in Escherichia coli cells containing functional Lon elicits specific phenotypes associated with lon deficiency, including the inhibition of persister cell formation. These findings uncover a substrate-dependent assembly process for the activation of a AAA+ protein and demonstrate a targeted approach to selectively inhibit its function within cells. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34000.map.gz emd_34000.map.gz | 107 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34000-v30.xml emd-34000-v30.xml emd-34000.xml emd-34000.xml | 18.3 KB 18.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34000.png emd_34000.png | 110.7 KB | ||
| Filedesc metadata |  emd-34000.cif.gz emd-34000.cif.gz | 6.5 KB | ||
| Others |  emd_34000_half_map_1.map.gz emd_34000_half_map_1.map.gz emd_34000_half_map_2.map.gz emd_34000_half_map_2.map.gz | 200.7 MB 200.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34000 http://ftp.pdbj.org/pub/emdb/structures/EMD-34000 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34000 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34000 | HTTPS FTP |
-Related structure data
| Related structure data |  7yphMC  7ypiC  7ypjC  7ypkC  7yuhC  7yumC  7yupC  7yutC  7yuuC  7yuvC  7yuwC  7yuxC  8k3yC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34000.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34000.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Pentameric state of MtaLon-Y224S with ATPgammaS | ||||||||||||||||||||||||||||||||||||
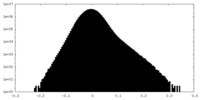
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map A
| File | emd_34000_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
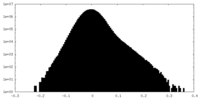
| Projections & Slices |
| ||||||||||||
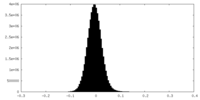
| Density Histograms |
-Half map: Half map B
| File | emd_34000_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
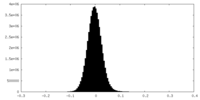
| Density Histograms |
- Sample components
Sample components
-Entire : Homo-pentameric complex of Lon protease with ATPgammaS
| Entire | Name: Homo-pentameric complex of Lon protease with ATPgammaS |
|---|---|
| Components |
|
-Supramolecule #1: Homo-pentameric complex of Lon protease with ATPgammaS
| Supramolecule | Name: Homo-pentameric complex of Lon protease with ATPgammaS type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Meiothermus taiwanensis (bacteria) Meiothermus taiwanensis (bacteria) |
| Molecular weight | Theoretical: 440 KDa |
-Macromolecule #1: Lon protease
| Macromolecule | Name: Lon protease / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO / EC number: endopeptidase La |
|---|---|
| Source (natural) | Organism:  Meiothermus taiwanensis (bacteria) Meiothermus taiwanensis (bacteria) |
| Molecular weight | Theoretical: 88.478492 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRLELPVIPL RNTVILPHTT TPVDVGRAKS KRAVEEAMGA DRLIFLVAQR DPEVDDPAPD DLYTWGVQAV VKQAMRLPDG TLQVMVEAR ARAQVTDYIP GPYLRARGEV FSEIFPIDEA VVRVLVEELK EAFEKYVANH KSLRLDRYQL EAVKGTSDPA M LADTIAYH ...String: MRLELPVIPL RNTVILPHTT TPVDVGRAKS KRAVEEAMGA DRLIFLVAQR DPEVDDPAPD DLYTWGVQAV VKQAMRLPDG TLQVMVEAR ARAQVTDYIP GPYLRARGEV FSEIFPIDEA VVRVLVEELK EAFEKYVANH KSLRLDRYQL EAVKGTSDPA M LADTIAYH ATWTVAEKQE ILELTDLEAR LKKVLGLLSR DLERFELDKR VAQRVKEQMD TNQRESYLRE QMKAIQKELG GE DGLSDLE ALRKKIEEVG MPEAVKTKAL KELDRLERMQ QGSPEATVAR TYLDWLTEVP WSKADPEVLD INHTRQVLDE DHY GLKDVK ERILEYLAVR QLTQGLDVRN KAPILVLVGP PGVGKTSLGR SIARSMNRKF HRISLGGVRD EAEIRGHRRT YIGA MPGKL IHAMKQVGVI NPVILLDEID KMSSDWRGDP ASAMLEVLDP EQNNTFTDHY LDVPYDLSKV FFITTANTLQ TIPRP LLDR MEVIEIPGYT NMEKQAIARQ YLWPKQVRES GMEGRIEVTD AAILRVISEY TREAGVRGLE RELGKIARKG AKFWLE GAW EGLRTIDASD IPTYLGIPRY RPDKAETEPQ VGTAQGLAWT PVGGTLLTIE VAAVPGSGKL SLTGQLGEVM KESAQAA LT YLRAHTQDYG LPEDFYNKVD LHVHVPDGAT PKDGPSAGIT MATAIASALS RRPARMDIAM TGEVSLRGKV MPIGGVKE K LLAAHQAGIH KIVLPKDNEA QLEELPKEVL EGLEIKLVED VGEVLEYLLL PEPTMPPVVQ PSDNRQQPGA GA UniProtKB: Lon protease |
-Macromolecule #2: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER / type: ligand / ID: 2 / Number of copies: 2 / Formula: AGS |
|---|---|
| Molecular weight | Theoretical: 523.247 Da |
| Chemical component information |  ChemComp-AGS: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K | ||||||||||||||||||
| Details | MtaLon-Y224S was incubated with ATPgammaS |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 2 / Number real images: 12982 / Average exposure time: 1.34 sec. / Average electron dose: 73.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 105000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)