+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cryo-EM structure of the glucagon receptor bound to beta-arrestin 1 in ligand-free state | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | Complex structure / glucagon receptor / beta-arrestin 1 / ligand-free / MEMBRANE PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報activated protein C (thrombin-activated peptidase) / positive regulation of establishment of endothelial barrier / renal water retention / Defective AVP does not bind AVPR2 and causes neurohypophyseal diabetes insipidus (NDI) / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin / vasopressin receptor activity / negative regulation of coagulation / regulation of glycogen metabolic process / hemostasis ...activated protein C (thrombin-activated peptidase) / positive regulation of establishment of endothelial barrier / renal water retention / Defective AVP does not bind AVPR2 and causes neurohypophyseal diabetes insipidus (NDI) / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin / vasopressin receptor activity / negative regulation of coagulation / regulation of glycogen metabolic process / hemostasis / glucagon receptor activity / telencephalon development / response to starvation / positive regulation of systemic arterial blood pressure / peptide hormone binding / positive regulation of intracellular signal transduction / endocytic vesicle / negative regulation of blood coagulation / Transport of gamma-carboxylated protein precursors from the endoplasmic reticulum to the Golgi apparatus / Gamma-carboxylation of protein precursors / Common Pathway of Fibrin Clot Formation / Removal of aminoterminal propeptides from gamma-carboxylated proteins / cellular response to hormone stimulus / positive regulation of vasoconstriction / activation of adenylate cyclase activity / Intrinsic Pathway of Fibrin Clot Formation / response to cytokine / response to nutrient / cellular response to glucagon stimulus / viral budding from plasma membrane / guanyl-nucleotide exchange factor activity / cellular response to starvation / generation of precursor metabolites and energy / Cell surface interactions at the vascular wall / Post-translational protein phosphorylation / clathrin-coated endocytic vesicle membrane / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / regulation of blood pressure / negative regulation of inflammatory response / Golgi lumen / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / Glucagon signaling in metabolic regulation / Glucagon-type ligand receptors / blood coagulation / Vasopressin regulates renal water homeostasis via Aquaporins / glucose homeostasis / Cargo recognition for clathrin-mediated endocytosis / Clathrin-mediated endocytosis / G alpha (s) signalling events / clathrin-dependent endocytosis of virus by host cell / G alpha (q) signalling events / cell surface receptor signaling pathway / endosome / host cell surface receptor binding / G protein-coupled receptor signaling pathway / endoplasmic reticulum lumen / negative regulation of cell population proliferation / fusion of virus membrane with host plasma membrane / serine-type endopeptidase activity / fusion of virus membrane with host endosome membrane / positive regulation of cell population proliferation / viral envelope / calcium ion binding / positive regulation of gene expression / negative regulation of apoptotic process / virion attachment to host cell / perinuclear region of cytoplasm / host cell plasma membrane / virion membrane / endoplasmic reticulum / Golgi apparatus / proteolysis / extracellular space / extracellular region / identical protein binding / membrane / plasma membrane 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) / Homo sapiens (ヒト) /   Escherichia phage EcSzw-2 (ファージ) Escherichia phage EcSzw-2 (ファージ) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.5 Å | |||||||||
 データ登録者 データ登録者 | Chen K / Zhang C / Lin S / Zhao Q / Wu B | |||||||||
| 資金援助 |  中国, 2件 中国, 2件
| |||||||||
 引用 引用 |  ジャーナル: Nature / 年: 2023 ジャーナル: Nature / 年: 2023タイトル: Tail engagement of arrestin at the glucagon receptor. 著者: Kun Chen / Chenhui Zhang / Shuling Lin / Xinyu Yan / Heng Cai / Cuiying Yi / Limin Ma / Xiaojing Chu / Yuchen Liu / Ya Zhu / Shuo Han / Qiang Zhao / Beili Wu /  要旨: Arrestins have pivotal roles in regulating G protein-coupled receptor (GPCR) signalling by desensitizing G protein activation and mediating receptor internalization. It has been proposed that the ...Arrestins have pivotal roles in regulating G protein-coupled receptor (GPCR) signalling by desensitizing G protein activation and mediating receptor internalization. It has been proposed that the arrestin binds to the receptor in two different conformations, 'tail' and 'core', which were suggested to govern distinct processes of receptor signalling and trafficking. However, little structural information is available for the tail engagement of the arrestins. Here we report two structures of the glucagon receptor (GCGR) bound to β-arrestin 1 (βarr1) in glucagon-bound and ligand-free states. These structures reveal a receptor tail-engaged binding mode of βarr1 with many unique features, to our knowledge, not previously observed. Helix VIII, instead of the receptor core, has a major role in accommodating βarr1 by forming extensive interactions with the central crest of βarr1. The tail-binding pose is further defined by a close proximity between the βarr1 C-edge and the receptor helical bundle, and stabilized by a phosphoinositide derivative that bridges βarr1 with helices I and VIII of GCGR. Lacking any contact with the arrestin, the receptor core is in an inactive state and loosely binds to glucagon. Further functional studies suggest that the tail conformation of GCGR-βarr governs βarr recruitment at the plasma membrane and endocytosis of GCGR, and provides a molecular basis for the receptor forming a super-complex simultaneously with G protein and βarr to promote sustained signalling within endosomes. These findings extend our knowledge about the arrestin-mediated modulation of GPCR functionalities. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_36606.map.gz emd_36606.map.gz | 59.5 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-36606-v30.xml emd-36606-v30.xml emd-36606.xml emd-36606.xml | 21.8 KB 21.8 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_36606.png emd_36606.png | 47.7 KB | ||
| Filedesc metadata |  emd-36606.cif.gz emd-36606.cif.gz | 7.3 KB | ||
| その他 |  emd_36606_half_map_1.map.gz emd_36606_half_map_1.map.gz emd_36606_half_map_2.map.gz emd_36606_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36606 http://ftp.pdbj.org/pub/emdb/structures/EMD-36606 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36606 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36606 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_36606_validation.pdf.gz emd_36606_validation.pdf.gz | 842.4 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_36606_full_validation.pdf.gz emd_36606_full_validation.pdf.gz | 842 KB | 表示 | |
| XML形式データ |  emd_36606_validation.xml.gz emd_36606_validation.xml.gz | 12.3 KB | 表示 | |
| CIF形式データ |  emd_36606_validation.cif.gz emd_36606_validation.cif.gz | 14.5 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36606 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36606 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36606 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36606 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  8jruMC  8jrvC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_36606.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_36606.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.071 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: #2
| ファイル | emd_36606_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
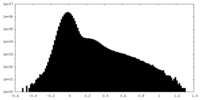
| 投影像・断面図 |
| ||||||||||||
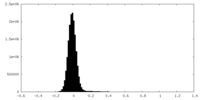
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_36606_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
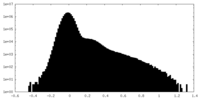
| 投影像・断面図 |
| ||||||||||||
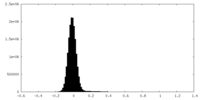
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : The glucagon receptor bound to beta-arrestin 1
| 全体 | 名称: The glucagon receptor bound to beta-arrestin 1 |
|---|---|
| 要素 |
|
-超分子 #1: The glucagon receptor bound to beta-arrestin 1
| 超分子 | 名称: The glucagon receptor bound to beta-arrestin 1 / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#3 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: HA signal peptide,HPC4 purification tag,Glucagon receptor,C-termi...
| 分子 | 名称: HA signal peptide,HPC4 purification tag,Glucagon receptor,C-terminal tail of Vasopressin V2 receptor タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 54.307141 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: GSMKTIIALS YIFCLVFAGA PEDQVDPRLI DGKGSGSAGS AGSQVMDFLF EKWKLYGDQC HHNLSLLPPP TELVCNRTFD KYSCWPDTP ANTTANISCP WYLPWHHKVQ HRFVFKRCGP DGQWVRGPRG QPWRDASQCQ MDGEEIEVQK EVAKMYSSFQ V MYTVGYSL ...文字列: GSMKTIIALS YIFCLVFAGA PEDQVDPRLI DGKGSGSAGS AGSQVMDFLF EKWKLYGDQC HHNLSLLPPP TELVCNRTFD KYSCWPDTP ANTTANISCP WYLPWHHKVQ HRFVFKRCGP DGQWVRGPRG QPWRDASQCQ MDGEEIEVQK EVAKMYSSFQ V MYTVGYSL SLGALLLALA ILGGLSKLHC TRNAIHANLF ASFVLKASSV LVIDGLLRTR YSQKIGDDLS VSTWLSDGAV AG CRVAAVF MQYGIVANYC WLLVEGLYLH NLLGLATLPE RSFFSLYLGI GWGAPMLFVV PWAVVKCLFE NVQCWTSNDN MGF WWILRF PVFLAILINF FIFVRIVQLL VAKLRARQMH HTDYKFRLAK STLTLIPLLG VHEVVFAFVT DEHAQGTLRS AKLF FDLFL SSFQGLLVAV LYCFLNKEVQ SELRRRWHRW RLGKVLWEER NTSNARGRTP PSLGPQDE(SEP)C T(TPO)A (SEP)(SEP)(SEP)LAK DTSS UniProtKB: Hemagglutinin, Vitamin K-dependent protein C, Glucagon receptor, Vasopressin V2 receptor |
-分子 #2: Beta-arrestin 1 and single-chain fragment variable 30 (scFv30)
| 分子 | 名称: Beta-arrestin 1 and single-chain fragment variable 30 (scFv30) タイプ: protein_or_peptide / ID: 2 / コピー数: 3 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 69.173891 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MGDKGTRVFK KASPNGKLTV YLGKRDFVDH IDLVEPVDGV VLVDPEYLKE RRVYVTLTAA FRYGREDLDV LGLTFRKDLF VANVQSFPP APEDKKPLTR LQERLIKKLG EHAYPFTFEI PPNLPSSVTL QPGPEDTGKA IGVDYEVKAF VAENLEEKIH K RNSVRLVI ...文字列: MGDKGTRVFK KASPNGKLTV YLGKRDFVDH IDLVEPVDGV VLVDPEYLKE RRVYVTLTAA FRYGREDLDV LGLTFRKDLF VANVQSFPP APEDKKPLTR LQERLIKKLG EHAYPFTFEI PPNLPSSVTL QPGPEDTGKA IGVDYEVKAF VAENLEEKIH K RNSVRLVI EKVQYAPERP GPQPTAETTR QFLMSDKPLH LEASLDKEIY YHGEPISVNV HVTNNTNKTV KKIKISVRQY AD IVLFNTA QYKVPVAMEE ADDTVAPSST FSKVYTLTPF LANNREKRGL ALDGKLKHED TNLASSTLLR EGANREILGI IVS YKVKVK LVVSRGGLLG DLASSDVAVE LPFTLMHPKP KEEPPHREVP EHETPVDTNL SDIQMTQSPS SLSASVGDRV TITC RASQS VSSAVAWYQQ KPGKAPKLLI YSASSLYSGV PSRFSGSRSG TDFTLTISSL QPEDFATYYC QQYKYVPVTF GQGTK VEIK GTTAASGSSG GSSSGAEVQL VESGGGLVQP GGSLRLSCAA SGFNVYSSSI HWVRQAPGKG LEWVASISSY YGYTYY ADS VKGRFTISAD TSKNTAYLQM NSLRAEDTAV YYCARSRQFW YSGLDYWGQG TLVTVSSAHH HHHH |
-分子 #3: Nanobody 32
| 分子 | 名称: Nanobody 32 / タイプ: protein_or_peptide / ID: 3 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Escherichia phage EcSzw-2 (ファージ) Escherichia phage EcSzw-2 (ファージ) |
| 分子量 | 理論値: 13.867408 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MAQVQLQESG GGLVQAGGSL RLSCVVSGFF FDTVTMAWYR RAPGKHRELV ASATAGGTTT YADSVKDRFT ISRDNAKNTV YLQMNSLKP EDTAVYYCNT FVRSLSWGQG TQVTVSSHHH HHHEPEA |
-分子 #4: [(2R)-2-octanoyloxy-3-[oxidanyl-[(1R,2R,3S,4R,5R,6S)-2,3,6-tris(o...
| 分子 | 名称: [(2R)-2-octanoyloxy-3-[oxidanyl-[(1R,2R,3S,4R,5R,6S)-2,3,6-tris(oxidanyl)-4,5-diphosphonooxy-cyclohexyl]oxy-phosphoryl]oxy-propyl] octanoate タイプ: ligand / ID: 4 / コピー数: 1 / 式: PIO |
|---|---|
| 分子量 | 理論値: 746.566 Da |
| Chemical component information |  ChemComp-PIO: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 6 mg/mL |
|---|---|
| 緩衝液 | pH: 7.5 |
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) 平均電子線量: 70.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: SPOT SCAN / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 1.5 µm / 最小 デフォーカス(公称値): 0.8 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)