[English] 日本語
 Yorodumi
Yorodumi- EMDB-36443: The cryo-EM structure of the decameric RAD51 ring bound to the nu... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | The cryo-EM structure of the decameric RAD51 ring bound to the nucleosome without the linker DNA binding | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Nucleosome / Recombinase / DNA BINDING PROTEIN-DNA complex | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationpresynaptic intermediate filament cytoskeleton / response to glucoside / mitotic recombination-dependent replication fork processing / DNA recombinase assembly / cellular response to camptothecin / chromosome organization involved in meiotic cell cycle / telomere maintenance via telomere lengthening / double-strand break repair involved in meiotic recombination / nuclear ubiquitin ligase complex / cellular response to cisplatin ...presynaptic intermediate filament cytoskeleton / response to glucoside / mitotic recombination-dependent replication fork processing / DNA recombinase assembly / cellular response to camptothecin / chromosome organization involved in meiotic cell cycle / telomere maintenance via telomere lengthening / double-strand break repair involved in meiotic recombination / nuclear ubiquitin ligase complex / cellular response to cisplatin / DNA strand invasion / cellular response to hydroxyurea / mitotic recombination / DNA strand exchange activity / replication-born double-strand break repair via sister chromatid exchange / lateral element / regulation of DNA damage checkpoint / Impaired BRCA2 binding to PALB2 / telomere maintenance via recombination / single-stranded DNA helicase activity / reciprocal meiotic recombination / Homologous DNA Pairing and Strand Exchange / Defective homologous recombination repair (HRR) due to BRCA1 loss of function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA1 binding function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA2/RAD51/RAD51C binding function / Resolution of D-loop Structures through Synthesis-Dependent Strand Annealing (SDSA) / Resolution of D-loop Structures through Holliday Junction Intermediates / HDR through Single Strand Annealing (SSA) / regulation of double-strand break repair via homologous recombination / ATP-dependent DNA damage sensor activity / nuclear chromosome / Impaired BRCA2 binding to RAD51 / Transcriptional Regulation by E2F6 / replication fork processing / Presynaptic phase of homologous DNA pairing and strand exchange / negative regulation of tumor necrosis factor-mediated signaling pathway / response to X-ray / ATP-dependent activity, acting on DNA / interstrand cross-link repair / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / Packaging Of Telomere Ends / condensed chromosome / DNA polymerase binding / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / telomere organization / Interleukin-7 signaling / Inhibition of DNA recombination at telomere / RNA Polymerase I Promoter Opening / Meiotic synapsis / Assembly of the ORC complex at the origin of replication / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / SUMOylation of chromatin organization proteins / DNA methylation / epigenetic regulation of gene expression / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / innate immune response in mucosa / condensed nuclear chromosome / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / cellular response to ionizing radiation / Defective pyroptosis / meiotic cell cycle / male germ cell nucleus / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / Nonhomologous End-Joining (NHEJ) / lipopolysaccharide binding / RNA Polymerase I Promoter Escape / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / cellular response to gamma radiation / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / double-strand break repair via homologous recombination / HDMs demethylate histones / G2/M DNA damage checkpoint / PML body / NoRC negatively regulates rRNA expression / DNA Damage/Telomere Stress Induced Senescence / HDR through Homologous Recombination (HRR) / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / Meiotic recombination / Pre-NOTCH Transcription and Translation / response to toxic substance / Metalloprotease DUBs / RMTs methylate histone arginines / Activation of anterior HOX genes in hindbrain development during early embryogenesis Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | ||||||||||||||||||
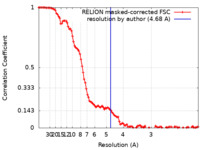
| Method | single particle reconstruction / cryo EM / Resolution: 4.68 Å | ||||||||||||||||||
 Authors Authors | Shioi T / Hatazawa S / Ogasawara M / Takizawa Y / Kurumizaka H | ||||||||||||||||||
| Funding support |  Japan, 5 items Japan, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Cryo-EM structures of RAD51 assembled on nucleosomes containing a DSB site. Authors: Takuro Shioi / Suguru Hatazawa / Eriko Oya / Noriko Hosoya / Wataru Kobayashi / Mitsuo Ogasawara / Takehiko Kobayashi / Yoshimasa Takizawa / Hitoshi Kurumizaka /  Abstract: RAD51 is the central eukaryotic recombinase required for meiotic recombination and mitotic repair of double-strand DNA breaks (DSBs). However, the mechanism by which RAD51 functions at DSB sites in ...RAD51 is the central eukaryotic recombinase required for meiotic recombination and mitotic repair of double-strand DNA breaks (DSBs). However, the mechanism by which RAD51 functions at DSB sites in chromatin has remained elusive. Here we report the cryo-electron microscopy structures of human RAD51-nucleosome complexes, in which RAD51 forms ring and filament conformations. In the ring forms, the N-terminal lobe domains (NLDs) of RAD51 protomers are aligned on the outside of the RAD51 ring, and directly bind to the nucleosomal DNA. The nucleosomal linker DNA that contains the DSB site is recognized by the L1 and L2 loops-active centres that face the central hole of the RAD51 ring. In the filament form, the nucleosomal DNA is peeled by the RAD51 filament extension, and the NLDs of RAD51 protomers proximal to the nucleosome bind to the remaining nucleosomal DNA and histones. Mutations that affect nucleosome-binding residues of the RAD51 NLD decrease nucleosome binding, but barely affect DNA binding in vitro. Consistently, yeast Rad51 mutants with the corresponding mutations are substantially defective in DNA repair in vivo. These results reveal an unexpected function of the RAD51 NLD, and explain the mechanism by which RAD51 associates with nucleosomes, recognizes DSBs and forms the active filament in chromatin. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36443.map.gz emd_36443.map.gz | 12.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36443-v30.xml emd-36443-v30.xml emd-36443.xml emd-36443.xml | 21.8 KB 21.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36443_fsc.xml emd_36443_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_36443.png emd_36443.png | 115.1 KB | ||
| Filedesc metadata |  emd-36443.cif.gz emd-36443.cif.gz | 6.6 KB | ||
| Others |  emd_36443_half_map_1.map.gz emd_36443_half_map_1.map.gz emd_36443_half_map_2.map.gz emd_36443_half_map_2.map.gz | 80.8 MB 80.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36443 http://ftp.pdbj.org/pub/emdb/structures/EMD-36443 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36443 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36443 | HTTPS FTP |
-Validation report
| Summary document |  emd_36443_validation.pdf.gz emd_36443_validation.pdf.gz | 839.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36443_full_validation.pdf.gz emd_36443_full_validation.pdf.gz | 839.5 KB | Display | |
| Data in XML |  emd_36443_validation.xml.gz emd_36443_validation.xml.gz | 17.7 KB | Display | |
| Data in CIF |  emd_36443_validation.cif.gz emd_36443_validation.cif.gz | 23.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36443 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36443 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36443 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36443 | HTTPS FTP |
-Related structure data
| Related structure data |  8jneMC  8jndC  8jnfC  8xbtC  8xbuC  8xbvC  8xbwC  8xbxC  8xbyC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36443.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36443.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36443_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
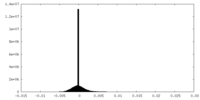
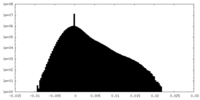
| Density Histograms |
-Half map: #1
| File | emd_36443_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
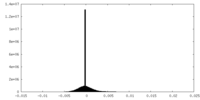
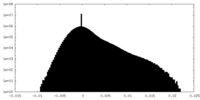
| Density Histograms |
- Sample components
Sample components
-Entire : RAD51-nucleosome complex
| Entire | Name: RAD51-nucleosome complex |
|---|---|
| Components |
|
-Supramolecule #1: RAD51-nucleosome complex
| Supramolecule | Name: RAD51-nucleosome complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#7 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Histone H3.1
| Macromolecule | Name: Histone H3.1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.719445 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSHMARTKQT ARKSTGGKAP RKQLATKAAR KSAPATGGVK KPHRYRPGTV ALREIRRYQK STELLIRKLP FQRLVREIAQ DFKTDLRFQ SSAVMALQEA CEAYLVGLFE DTNLCAIHAK RVTIMPKDIQ LARRIRGERA UniProtKB: Histone H3.1 |
-Macromolecule #2: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.676703 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSHMSGRGKG GKGLGKGGAK RHRKVLRDNI QGITKPAIRR LARRGGVKRI SGLIYEETRG VLKVFLENVI RDAVTYTEHA KRKTVTAMD VVYALKRQGR TLYGFGG UniProtKB: Histone H4 |
-Macromolecule #3: Histone H2A type 1-B/E
| Macromolecule | Name: Histone H2A type 1-B/E / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 14.447825 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSHMSGRGKQ GGKARAKAKT RSSRAGLQFP VGRVHRLLRK GNYSERVGAG APVYLAAVLE YLTAEILELA GNAARDNKKT RIIPRHLQL AIRNDEELNK LLGRVTIAQG GVLPNIQAVL LPKKTESHHK AKGK UniProtKB: Histone H2A type 1-B/E |
-Macromolecule #4: Histone H2B type 1-J
| Macromolecule | Name: Histone H2B type 1-J / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 14.217516 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSHMPEPAKS APAPKKGSKK AVTKAQKKDG KKRKRSRKES YSIYVYKVLK QVHPDTGISS KAMGIMNSFV NDIFERIAGE ASRLAHYNK RSTITSREIQ TAVRLLLPGE LAKHAVSEGT KAVTKYTSAK UniProtKB: Histone H2B type 1-J |
-Macromolecule #7: DNA repair protein RAD51 homolog 1
| Macromolecule | Name: DNA repair protein RAD51 homolog 1 / type: protein_or_peptide / ID: 7 / Number of copies: 10 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.291398 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSHMAMQMQL EANADTSVEE ESFGPQPISR LEQCGINAND VKKLEEAGFH TVEAVAYAPK KELINIKGIS EAKADKILAE AAKLVPMGF TTATEFHQRR SEIIQITTGS KELDKLLQGG IETGSITEMF GEFRTGKTQI CHTLAVTCQL PIDRGGGEGK A MYIDTEGT ...String: GSHMAMQMQL EANADTSVEE ESFGPQPISR LEQCGINAND VKKLEEAGFH TVEAVAYAPK KELINIKGIS EAKADKILAE AAKLVPMGF TTATEFHQRR SEIIQITTGS KELDKLLQGG IETGSITEMF GEFRTGKTQI CHTLAVTCQL PIDRGGGEGK A MYIDTEGT FRPERLLAVA ERYGLSGSDV LDNVAYARAF NTDHQTQLLY QASAMMVESR YALLIVDSAT ALYRTDYSGR GE LSARQMH LARFLRMLLR LADEFGVAVV ITNQVVAQVD GAAMFAADPK KPIGGNIIAH ASTTRLYLRK GRGETRICKI YDS PCLPEA EAMFAINADG VGDAKD UniProtKB: DNA repair protein RAD51 homolog 1 |
-Macromolecule #5: DNA (156-MER)
| Macromolecule | Name: DNA (156-MER) / type: dna / ID: 5 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 47.976699 KDa |
| Sequence | String: (DA)(DT)(DC)(DA)(DG)(DA)(DA)(DT)(DC)(DC) (DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA)(DG) (DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA)(DA) (DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA)(DG) (DA) (DC)(DA)(DG)(DC)(DT)(DC) ...String: (DA)(DT)(DC)(DA)(DG)(DA)(DA)(DT)(DC)(DC) (DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA)(DG) (DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA)(DA) (DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA)(DG) (DA) (DC)(DA)(DG)(DC)(DT)(DC)(DT)(DA) (DG)(DC)(DA)(DC)(DC)(DG)(DC)(DT)(DT)(DA) (DA)(DA) (DC)(DG)(DC)(DA)(DC)(DG)(DT) (DA)(DC)(DG)(DC)(DG)(DC)(DT)(DG)(DT)(DC) (DC)(DC)(DC) (DC)(DG)(DC)(DG)(DT)(DT) (DT)(DT)(DA)(DA)(DC)(DC)(DG)(DC)(DC)(DA) (DA)(DG)(DG)(DG) (DG)(DA)(DT)(DT)(DA) (DC)(DA)(DC)(DC)(DC)(DA)(DA)(DG)(DA)(DC) (DA)(DC)(DC)(DA)(DG) (DG)(DC)(DA)(DC) (DG)(DA)(DG)(DA)(DC)(DA)(DG)(DA)(DA)(DA) (DA)(DA)(DA)(DA)(DC)(DA) (DA)(DC)(DG) (DA)(DA)(DA)(DA)(DC)(DG)(DG)(DC)(DC)(DA) (DC)(DC)(DA) |
-Macromolecule #6: DNA (153-MER)
| Macromolecule | Name: DNA (153-MER) / type: dna / ID: 6 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 47.37107 KDa |
| Sequence | String: (DT)(DG)(DG)(DC)(DC)(DG)(DT)(DT)(DT)(DT) (DC)(DG)(DT)(DT)(DG)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DC)(DT)(DG)(DT)(DC)(DT)(DC) (DG)(DT)(DG)(DC)(DC)(DT)(DG)(DG)(DT)(DG) (DT) (DC)(DT)(DT)(DG)(DG)(DG) ...String: (DT)(DG)(DG)(DC)(DC)(DG)(DT)(DT)(DT)(DT) (DC)(DG)(DT)(DT)(DG)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DC)(DT)(DG)(DT)(DC)(DT)(DC) (DG)(DT)(DG)(DC)(DC)(DT)(DG)(DG)(DT)(DG) (DT) (DC)(DT)(DT)(DG)(DG)(DG)(DT)(DG) (DT)(DA)(DA)(DT)(DC)(DC)(DC)(DC)(DT)(DT) (DG)(DG) (DC)(DG)(DG)(DT)(DT)(DA)(DA) (DA)(DA)(DC)(DG)(DC)(DG)(DG)(DG)(DG)(DG) (DA)(DC)(DA) (DG)(DC)(DG)(DC)(DG)(DT) (DA)(DC)(DG)(DT)(DG)(DC)(DG)(DT)(DT)(DT) (DA)(DA)(DG)(DC) (DG)(DG)(DT)(DG)(DC) (DT)(DA)(DG)(DA)(DG)(DC)(DT)(DG)(DT)(DC) (DT)(DA)(DC)(DG)(DA) (DC)(DC)(DA)(DA) (DT)(DT)(DG)(DA)(DG)(DC)(DG)(DG)(DC)(DC) (DT)(DC)(DG)(DG)(DC)(DA) (DC)(DC)(DG) (DG)(DG)(DA)(DT)(DT)(DC)(DT)(DG)(DA)(DT) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)