[English] 日本語
 Yorodumi
Yorodumi- EMDB-36233: CryoEM structure of Gi-coupled MRGPRX1 with peptide agonist BAM8-22 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of Gi-coupled MRGPRX1 with peptide agonist BAM8-22 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | itch receptor / Mas-related GPCRs / MRGPRX1 / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsynaptic vesicle lumen / response to chloroquine / chromaffin granule lumen / opioid peptide activity / aggressive behavior / positive regulation of behavioral fear response / general adaptation syndrome, behavioral process / symmetric synapse / neuronal dense core vesicle lumen / synaptic signaling via neuropeptide ...synaptic vesicle lumen / response to chloroquine / chromaffin granule lumen / opioid peptide activity / aggressive behavior / positive regulation of behavioral fear response / general adaptation syndrome, behavioral process / symmetric synapse / neuronal dense core vesicle lumen / synaptic signaling via neuropeptide / response to epinephrine / cell body fiber / G protein-coupled opioid receptor signaling pathway / cellular response to vitamin D / sensory perception / neuropeptide hormone activity / startle response / response to immobilization stress / transmission of nerve impulse / locomotory exploration behavior / cellular response to transforming growth factor beta stimulus / neuropeptide signaling pathway / behavioral fear response / glial cell proliferation / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / axon terminus / adenylate cyclase regulator activity / G protein-coupled serotonin receptor binding / adenylate cyclase-inhibiting serotonin receptor signaling pathway / sensory perception of pain / cellular response to forskolin / cellular response to cAMP / Peptide ligand-binding receptors / regulation of mitotic spindle organization / acute-phase response / response to nicotine / Post-translational protein phosphorylation / Regulation of insulin secretion / positive regulation of cholesterol biosynthetic process / response to calcium ion / negative regulation of insulin secretion / G protein-coupled receptor binding / response to peptide hormone / G protein-coupled receptor activity / cellular response to virus / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / response to toxic substance / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / osteoblast differentiation / Sensory perception of sweet, bitter, and umami (glutamate) taste / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / GDP binding / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / transmembrane signaling receptor activity / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / response to estradiol / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade / G alpha (12/13) signalling events / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / G protein activity / retina development in camera-type eye Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Sun JP / Xu HE / Yang F / Liu ZM / Guo LL / Zhang YM / Fang GX / Tie L / Zhuang YM / Xue CY | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Ligand recognition and G protein coupling of the human itch receptor MRGPRX1. Authors: Lulu Guo / Yumu Zhang / Guoxing Fang / Lu Tie / Yuming Zhuang / Chenyang Xue / Qi Liu / Minghui Zhang / Kongkai Zhu / Chongzhao You / Peiyu Xu / Qingning Yuan / Chao Zhang / Lei Liu / ...Authors: Lulu Guo / Yumu Zhang / Guoxing Fang / Lu Tie / Yuming Zhuang / Chenyang Xue / Qi Liu / Minghui Zhang / Kongkai Zhu / Chongzhao You / Peiyu Xu / Qingning Yuan / Chao Zhang / Lei Liu / Naikang Rong / Shengxuan Peng / Yuan Liu / Chuanzheng Wang / Xin Luo / Zongyao Lv / Dongwei Kang / Xiao Yu / Cheng Zhang / Yi Jiang / Xinzhong Dong / Jiuyao Zhou / Zhongmin Liu / Fan Yang / H Eric Xu / Jin-Peng Sun /   Abstract: MRGPRX1, a Mas-related GPCR (MRGPR), is a key receptor for itch perception and targeting MRGPRX1 may have potential to treat both chronic itch and pain. Here we report cryo-EM structures of the ...MRGPRX1, a Mas-related GPCR (MRGPR), is a key receptor for itch perception and targeting MRGPRX1 may have potential to treat both chronic itch and pain. Here we report cryo-EM structures of the MRGPRX1-Gi1 and MRGPRX1-Gq trimers in complex with two peptide ligands, BAM8-22 and CNF-Tx2. These structures reveal a shallow orthosteric pocket and its conformational plasticity for sensing multiple different peptidic itch allergens. Distinct from MRGPRX2, MRGPRX1 contains a unique pocket feature at the extracellular ends of TM3 and TM4 to accommodate the peptide C-terminal "RF/RY" motif, which could serve as key mechanisms for peptidic allergen recognition. Below the ligand binding pocket, the GXPFGXF/W motif is essential for the inward tilting of the upper end of TM6 to induce receptor activation. Moreover, structural features inside the ligand pocket and on the cytoplasmic side of MRGPRX1 are identified as key elements for both Gi and Gq signaling. Collectively, our studies provide structural insights into understanding itch sensation, MRGPRX1 activation, and downstream G protein signaling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36233.map.gz emd_36233.map.gz | 33 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36233-v30.xml emd-36233-v30.xml emd-36233.xml emd-36233.xml | 21 KB 21 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36233.png emd_36233.png | 89.2 KB | ||
| Filedesc metadata |  emd-36233.cif.gz emd-36233.cif.gz | 6.6 KB | ||
| Others |  emd_36233_half_map_1.map.gz emd_36233_half_map_1.map.gz emd_36233_half_map_2.map.gz emd_36233_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36233 http://ftp.pdbj.org/pub/emdb/structures/EMD-36233 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36233 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36233 | HTTPS FTP |
-Related structure data
| Related structure data |  8jggMC  8jgbC  8jgfC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36233.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36233.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.89 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36233_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
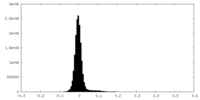
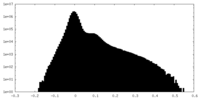
| Density Histograms |
-Half map: #1
| File | emd_36233_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
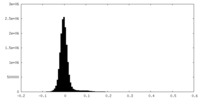
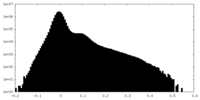
| Density Histograms |
- Sample components
Sample components
-Entire : CryoEM structure of Gi-coupled MRGPRX1 with peptide agonist BAM8-22
| Entire | Name: CryoEM structure of Gi-coupled MRGPRX1 with peptide agonist BAM8-22 |
|---|---|
| Components |
|
-Supramolecule #1: CryoEM structure of Gi-coupled MRGPRX1 with peptide agonist BAM8-22
| Supramolecule | Name: CryoEM structure of Gi-coupled MRGPRX1 with peptide agonist BAM8-22 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 6.633625 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: NTASIAQARK LVEQLKMEAN IDRIKVSKAA ADLMAYCEAH AKEDPLLTPV PASENPFREK UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #2: BAM8-22
| Macromolecule | Name: BAM8-22 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 1.603797 KDa |
| Sequence | String: PEWWMDYQKR Y UniProtKB: Proenkephalin-A |
-Macromolecule #3: Single-Chain Fragment Variable 16
| Macromolecule | Name: Single-Chain Fragment Variable 16 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 30.363043 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFAVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSAG G GGSGGGGS ...String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFAVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSAG G GGSGGGGS GGGGSADIVM TQATSSVPVT PGESVSISCR SSKSLLHSNG NTYLYWFLQR PGQSPQLLIY RMSNLASGVP DR FSGSGSG TAFTLTISRL EAEDVGVYYC MQHLEYPLTF GAGTKLEL |
-Macromolecule #4: Guanine nucleotide-binding protein G(i) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-1 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.415031 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKSTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKSTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTTGIVETH FTFKDLHFKM FDVGGQRSER KKWIHCFEGV TAIIFCVALS DYDLVLAEDE EM NRMHESM KLFDSICNNK WFTDTSIILF LNKKDLFEEK IKKSPLTICY PEYAGSNTYE EAAAYIQCQF EDLNKRKDTK EIY THFTCA TDTKNVQFVF DAVTDVIIKN NLKDCGLF UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1 |
-Macromolecule #5: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 38.561152 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: LEVLFQGPCG SSGSELDQLR QEAEQLKNQI RDARKACADA TLSQITNNID PVGRIQMRTR RTLRGHLAKI YAMHWGTDSR LLVSASQDG KLIIWDSYTT NKVHAIPLRS SWVMTCAYAP SGNYVACGGL DNICSIYNLK TREGNVRVSR ELAGHTGYLS C CRFLDDNQ ...String: LEVLFQGPCG SSGSELDQLR QEAEQLKNQI RDARKACADA TLSQITNNID PVGRIQMRTR RTLRGHLAKI YAMHWGTDSR LLVSASQDG KLIIWDSYTT NKVHAIPLRS SWVMTCAYAP SGNYVACGGL DNICSIYNLK TREGNVRVSR ELAGHTGYLS C CRFLDDNQ IVTSSGDTTC ALWDIETGQQ TTTFTGHTGD VMSLSLAPDT RLFVSGACDA SAKLWDVREG MCRQTFTGHE SD INAICFF PNGNAFATGS DDATCRLFDL RADQELMTYS HDNIICGITS VSFSKSGRLL LAGYDDFNCN VWDALKADRA GVL AGHDNR VSCLGVTDDG MAVATGSWDS FLKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #6: Mas-related G-protein coupled receptor member X1
| Macromolecule | Name: Mas-related G-protein coupled receptor member X1 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 36.276906 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDPTISTLDT ELTPINGTEE TLCYKQTLSL TVLTCIVSLV GLTGNAVVLW LLGCRMRRNA FSIYILNLAA ADFLFLSGRL IYSLLSFIS IPHTISKILY PVMMFSYFAG LSFLSAVSTE RCLSVLWPIW YRCHRPTHLS AVVCVLLWAL SLLRSILEWM L CGFLFSGA ...String: MDPTISTLDT ELTPINGTEE TLCYKQTLSL TVLTCIVSLV GLTGNAVVLW LLGCRMRRNA FSIYILNLAA ADFLFLSGRL IYSLLSFIS IPHTISKILY PVMMFSYFAG LSFLSAVSTE RCLSVLWPIW YRCHRPTHLS AVVCVLLWAL SLLRSILEWM L CGFLFSGA DSAWCQTSDF ITVAWLIFLC VVLCGSSLVL LIRILCGSRK IPLTRLYVTI LLTVLVFLLC GLPFGIQFFL FL WIHVDRE VLFCHVHLVS IFLSALNSSA NPIIYFFVGS FRQRQNRQNL KLVLQRALQD ASEVDEGGGQ LPEEILELSG SRL EQ UniProtKB: Mas-related G-protein coupled receptor member X1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DIFFRACTION / Nominal defocus max: 1.2 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 924644 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller



































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)