[English] 日本語
 Yorodumi
Yorodumi- EMDB-36166: Cryo-EM structure of mGlu2-mGlu3 heterodimer in presence of LY341... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of mGlu2-mGlu3 heterodimer in presence of LY341495 (dimerization mode II) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Complex structure / mGlu2-3 heterodimer / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of biosynthetic process / positive regulation of multicellular organismal process / regulation of response to drug / group II metabotropic glutamate receptor activity / regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / regulation of cellular response to stress / macrolide binding / TORC1 complex / activin receptor binding / regulation of skeletal muscle contraction by regulation of release of sequestered calcium ion ...positive regulation of biosynthetic process / positive regulation of multicellular organismal process / regulation of response to drug / group II metabotropic glutamate receptor activity / regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / regulation of cellular response to stress / macrolide binding / TORC1 complex / activin receptor binding / regulation of skeletal muscle contraction by regulation of release of sequestered calcium ion / intracellular glutamate homeostasis / cytoplasmic side of membrane / transforming growth factor beta receptor binding / behavioral response to nicotine / TGFBR1 LBD Mutants in Cancer / type I transforming growth factor beta receptor binding / signaling receptor inhibitor activity / negative regulation of adenylate cyclase activity / negative regulation of activin receptor signaling pathway / G protein-coupled glutamate receptor signaling pathway / heart trabecula formation / I-SMAD binding / Class C/3 (Metabotropic glutamate/pheromone receptors) / glutamate secretion / long-term synaptic depression / regulation of amyloid precursor protein catabolic process / glutamate receptor activity / terminal cisterna / ryanodine receptor complex / regulation of glutamate secretion / 'de novo' protein folding / astrocyte projection / ventricular cardiac muscle tissue morphogenesis / cellular response to stress / FK506 binding / regulation of cell size / regulation of dopamine secretion / TGF-beta receptor signaling activates SMADs / regulation of ryanodine-sensitive calcium-release channel activity / regulation of lipid metabolic process / mTORC1-mediated signalling / Calcineurin activates NFAT / regulation of immune response / regulation of synaptic transmission, glutamatergic / heart morphogenesis / postsynaptic modulation of chemical synaptic transmission / phagocytic vesicle / supramolecular fiber organization / sarcoplasmic reticulum membrane / presynaptic modulation of chemical synaptic transmission / negative regulation of autophagy / T cell activation / sarcoplasmic reticulum / TGF-beta receptor signaling in EMT (epithelial to mesenchymal transition) / response to cocaine / protein maturation / peptidylprolyl isomerase / calcium channel regulator activity / peptidyl-prolyl cis-trans isomerase activity / positive regulation of cell differentiation / negative regulation of transforming growth factor beta receptor signaling pathway / PML body / G protein-coupled receptor activity / Z disc / SARS-CoV-1 activates/modulates innate immune responses / regulation of protein localization / protein folding / protein refolding / presynaptic membrane / scaffold protein binding / gene expression / G alpha (i) signalling events / dendritic spine / amyloid fibril formation / Potential therapeutics for SARS / chemical synaptic transmission / transmembrane transporter binding / postsynaptic membrane / mitochondrial outer membrane / positive regulation of canonical NF-kappaB signal transduction / non-specific serine/threonine protein kinase / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / postsynaptic density / Golgi membrane / axon / lysosomal membrane / protein serine/threonine kinase activity / dendrite / endoplasmic reticulum membrane / protein-containing complex binding / glutamatergic synapse / ATP binding / membrane / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Wang X / Wang M / Xu T / Feng Y / Han S / Zhao Q / Wu B | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Cell Res / Year: 2023 Journal: Cell Res / Year: 2023Title: Structural insights into dimerization and activation of the mGlu2-mGlu3 and mGlu2-mGlu4 heterodimers. Authors: Xinwei Wang / Mu Wang / Tuo Xu / Ye Feng / Qiang Shao / Shuo Han / Xiaojing Chu / Yechun Xu / Shuling Lin / Qiang Zhao / Beili Wu /  Abstract: Heterodimerization of the metabotropic glutamate receptors (mGlus) has shown importance in the functional modulation of the receptors and offers potential drug targets for treating central nervous ...Heterodimerization of the metabotropic glutamate receptors (mGlus) has shown importance in the functional modulation of the receptors and offers potential drug targets for treating central nervous system diseases. However, due to a lack of molecular details of the mGlu heterodimers, understanding of the mechanisms underlying mGlu heterodimerization and activation is limited. Here we report twelve cryo-electron microscopy (cryo-EM) structures of the mGlu2-mGlu3 and mGlu2-mGlu4 heterodimers in different conformational states, including inactive, intermediate inactive, intermediate active and fully active conformations. These structures provide a full picture of conformational rearrangement of mGlu2-mGlu3 upon activation. The Venus flytrap domains undergo a sequential conformational change, while the transmembrane domains exhibit a substantial rearrangement from an inactive, symmetric dimer with diverse dimerization patterns to an active, asymmetric dimer in a conserved dimerization mode. Combined with functional data, these structures reveal that stability of the inactive conformations of the subunits and the subunit-G protein interaction pattern are determinants of asymmetric signal transduction of the heterodimers. Furthermore, a novel binding site for two mGlu4 positive allosteric modulators was observed in the asymmetric dimer interfaces of the mGlu2-mGlu4 heterodimer and mGlu4 homodimer, and may serve as a drug recognition site. These findings greatly extend our knowledge about signal transduction of the mGlus. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36166.map.gz emd_36166.map.gz | 168.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36166-v30.xml emd-36166-v30.xml emd-36166.xml emd-36166.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36166_fsc.xml emd_36166_fsc.xml | 2.3 MB | Display |  FSC data file FSC data file |
| Images |  emd_36166.png emd_36166.png | 61.4 KB | ||
| Filedesc metadata |  emd-36166.cif.gz emd-36166.cif.gz | 6.9 KB | ||
| Others |  emd_36166_half_map_1.map.gz emd_36166_half_map_1.map.gz emd_36166_half_map_2.map.gz emd_36166_half_map_2.map.gz | 165.3 MB 165.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36166 http://ftp.pdbj.org/pub/emdb/structures/EMD-36166 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36166 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36166 | HTTPS FTP |
-Related structure data
| Related structure data |  8jcvMC  8jcuC  8jcwC  8jcxC  8jcyC  8jczC  8jd0C  8jd1C  8jd2C  8jd3C  8jd4C  8jd5C  8jd6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36166.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36166.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.071 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36166_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_36166_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : mGlu2-3 heterodimer in presence of LY341495
| Entire | Name: mGlu2-3 heterodimer in presence of LY341495 |
|---|---|
| Components |
|
-Supramolecule #1: mGlu2-3 heterodimer in presence of LY341495
| Supramolecule | Name: mGlu2-3 heterodimer in presence of LY341495 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Metabotropic glutamate receptor 2,Peptidyl-prolyl cis-trans isome...
| Macromolecule | Name: Metabotropic glutamate receptor 2,Peptidyl-prolyl cis-trans isomerase FKBP1A type: protein_or_peptide / ID: 1 Details: Author stated 'all the data are processed in Cryosparc with default prameters, as all the other deposition. There should be no contamination.' Number of copies: 1 / Enantiomer: LEVO / EC number: peptidylprolyl isomerase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 109.340875 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DYKDDDDGAP EGPAKKVLTL EGDLVLGGLF PVHQKGGPAE DCGPVNEHRG IQRLEAMLFA LDRINRDPHL LPGVRLGAHI LDSCSKDTH ALEQALDFVR ASLSRGADGS RHICPDGSYA THGDAPTAIT GVIGGSYSDV SIQVANLLRL FQIPQISYAS T SAKLSDKS ...String: DYKDDDDGAP EGPAKKVLTL EGDLVLGGLF PVHQKGGPAE DCGPVNEHRG IQRLEAMLFA LDRINRDPHL LPGVRLGAHI LDSCSKDTH ALEQALDFVR ASLSRGADGS RHICPDGSYA THGDAPTAIT GVIGGSYSDV SIQVANLLRL FQIPQISYAS T SAKLSDKS RYDYFARTVP PDFFQAKAMA EILRFFNWTY VSTVASEGDY GETGIEAFEL EARARNICVA TSEKVGRAMS RA AFEGVVR ALLQKPSARV AVLFTRSEDA RELLAASQRL NASFTWVASD GWGALESVVA GSEGAAEGAI TIELASYPIS DFA SYFQSL DPWNNSRNPW FREFWEQRFR CSFRQRDCAA HSLRAVPFEQ ESKIMFVVNA VYAMAHALHN MHRALCPNTT RLCD AMRPV NGRRLYKDFV LNVKFDAPFR PADTHNEVRF DRFGDGIGRY NIFTYLRAGS GRYRYQKVGY WAEGLTLDTS LIPWA SPSA GPLPASRCSE PCLQNEVKSV QPGEVCCWLC IPCQPYEYRL DEFTCADCGL GYWPNASLTG CFALPQEYIR WGDAWA VGP VTIACLGALA TLFVLGVFVR HNATPVVKAS GRELCYILLG GVFLCYCMTF IFIAKPSTAV CTLRRLGLGT AFSVCYS AL LTKTNRIARI FGGAREGAQR PRFISPASQV AICLALISGQ LLIVVAWLVV EAPGTGKETA PERREVVTLR CNHRDASM L GSLAYNVLLI ALCTLYAFKT RKCPENFNEA KFIGFTMYTT CIIWLAFLPI FYVTSSDYRV QTTTMCVSVS LSGSVVLGC LFAPKLHIIL FQPQKNVVSH RAPTSRFGSA AARASSSLGQ GSGSQFVPTV CNGREVVDST TSSLLEVLFQ GPGVQVETIS PGDGRTFPK RGQTCVVHYT GMLEDGKKFD SSRDRNKPFK FMLGKQEVIR GWEEGVAQMS VGQRAKLTIS PDYAYGATGH P GIIPPHAT LVFDVELLKL EFAAAHHHHH HHHHH UniProtKB: Metabotropic glutamate receptor 2, Peptidyl-prolyl cis-trans isomerase FKBP1A |
-Macromolecule #2: Metabotropic glutamate receptor 3,Serine/threonine-protein kinase mTOR
| Macromolecule | Name: Metabotropic glutamate receptor 3,Serine/threonine-protein kinase mTOR type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: non-specific serine/threonine protein kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 112.712961 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DYKDDDDKGA PWSHPQFEKG SGSWSHPQFE KLGDHNFLRR EIKIEGDLVL GGLFPINEKG TGTEECGRIN EDRGIQRLEA MLFAIDEIN KDDYLLPGVK LGVHILDTCS RDTYALEQSL EFVRASLTKV DEAEYMCPDG SYAIQENIPL LIAGVIGGSY S SVSIQVAN ...String: DYKDDDDKGA PWSHPQFEKG SGSWSHPQFE KLGDHNFLRR EIKIEGDLVL GGLFPINEKG TGTEECGRIN EDRGIQRLEA MLFAIDEIN KDDYLLPGVK LGVHILDTCS RDTYALEQSL EFVRASLTKV DEAEYMCPDG SYAIQENIPL LIAGVIGGSY S SVSIQVAN LLRLFQIPQI SYASTSAKLS DKSRYDYFAR TVPPDFYQAK AMAEILRFFN WTYVSTVASE GDYGETGIEA FE QEARLRN ICIATAEKVG RSNIRKSYDS VIRELLQKPN ARVVVLFMRS DDSRELIAAA SRANASFTWV ASDGWGAQES IIK GSEHVA YGAITLELAS QPVRQFDRYF QSLNPYNNHR NPWFRDFWEQ KFQCSLQNKR NHRRVCDKHL AIDSSNYEQE SKIM FVVNA VYAMAHALHK MQRTLCPNTT KLCDAMKILD GKKLYKDYLL KINFTAPFNP NKDADSIVKF DTFGDGMGRY NVFNF QNVG GKYSYLKVGH WAETLSLDVN SIHWSRNSVP TSQCSDPCAP NEMKNMQPGD VCCWICIPCE PYEYLADEFT CMDCGS GQW PTADLTGCYD LPEDYIRWED AWAIGPVTIA CLGFMCTCMV VTVFIKHNNT PLVKASGREL CYILLFGVGL SYCMTFF FI AKPSPVICAL RRLGLGSSFA ICYSALLTKT NCIARIFDGV KNGAQRPKFI SPSSQVFICL GLILVQIVMV SVWLILEA P GTRRYTLAEK RETVILKCNV KDSSMLISLT YDVILVILCT VYAFKTRKCP ENFNEAKFIG FTMYTTCIIW LAFLPIFYV TSSDYRVQTT TMCISVSLSG FVVLGCLFAP KVHIILFQPQ KNVVTHRLHL NRFSVSGTGT TYSQSSASTY VPTVCNGREV LDSTTSSLL EVLFQGPAIL WHEMWHEGLE EASRLYFGER NVKGMFEVLE PLHAMMERGP QTLKETSFNQ AYGRDLMEAQ E WCRKYMKS GNVKDLTQAW DLYYHVFRRI SKQEF UniProtKB: Metabotropic glutamate receptor 3, Serine/threonine-protein kinase mTOR |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 2 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #4: 2-[(1S,2S)-2-carboxycyclopropyl]-3-(9H-xanthen-9-yl)-D-alanine
| Macromolecule | Name: 2-[(1S,2S)-2-carboxycyclopropyl]-3-(9H-xanthen-9-yl)-D-alanine type: ligand / ID: 4 / Number of copies: 2 / Formula: Z99 |
|---|---|
| Molecular weight | Theoretical: 353.369 Da |
| Chemical component information | 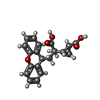 ChemComp-Z99: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)