[English] 日本語
 Yorodumi
Yorodumi- EMDB-35466: Semliki Forest virus VLP in complex with the receptor VLDLR-LA3 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Semliki Forest virus VLP in complex with the receptor VLDLR-LA3 | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
| Biological species |   Semliki Forest virus Semliki Forest virus | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.1 Å | ||||||||||||
 Authors Authors | Cao D / Ma B / Cao Z / Zhang X / Xiang Y | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Cell / Year: 2023 Journal: Cell / Year: 2023Title: Structure of Semliki Forest virus in complex with its receptor VLDLR. Authors: Duanfang Cao / Bingting Ma / Ziyi Cao / Xinzheng Zhang / Ye Xiang /  Abstract: Semliki Forest virus (SFV) is an alphavirus that uses the very-low-density lipoprotein receptor (VLDLR) as a receptor during infection of its vertebrate hosts and insect vectors. Herein, we used ...Semliki Forest virus (SFV) is an alphavirus that uses the very-low-density lipoprotein receptor (VLDLR) as a receptor during infection of its vertebrate hosts and insect vectors. Herein, we used cryoelectron microscopy to study the structure of SFV in complex with VLDLR. We found that VLDLR binds multiple E1-DIII sites of SFV through its membrane-distal LDLR class A (LA) repeats. Among the LA repeats of the VLDLR, LA3 has the best binding affinity to SFV. The high-resolution structure shows that LA3 binds SFV E1-DIII through a small surface area of 378 Å, with the main interactions at the interface involving salt bridges. Compared with the binding of single LA3s, consecutive LA repeats around LA3 promote synergistic binding to SFV, during which the LAs undergo a rotation, allowing simultaneous key interactions at multiple E1-DIII sites on the virion and enabling the binding of VLDLRs from divergent host species to SFV. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35466.map.gz emd_35466.map.gz | 802.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35466-v30.xml emd-35466-v30.xml emd-35466.xml emd-35466.xml | 12.5 KB 12.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35466.png emd_35466.png | 172.7 KB | ||
| Others |  emd_35466_half_map_1.map.gz emd_35466_half_map_1.map.gz emd_35466_half_map_2.map.gz emd_35466_half_map_2.map.gz | 795.1 MB 796.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35466 http://ftp.pdbj.org/pub/emdb/structures/EMD-35466 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35466 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35466 | HTTPS FTP |
-Validation report
| Summary document |  emd_35466_validation.pdf.gz emd_35466_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_35466_full_validation.pdf.gz emd_35466_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_35466_validation.xml.gz emd_35466_validation.xml.gz | 24.9 KB | Display | |
| Data in CIF |  emd_35466_validation.cif.gz emd_35466_validation.cif.gz | 29.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35466 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35466 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35466 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35466 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_35466.map.gz / Format: CCP4 / Size: 1.6 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35466.map.gz / Format: CCP4 / Size: 1.6 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
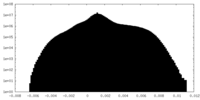
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_35466_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
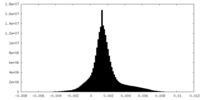
| Density Histograms |
-Half map: #1
| File | emd_35466_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
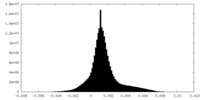
| Density Histograms |
- Sample components
Sample components
-Entire : Semliki Forest virus VLP in complex with the receptor VLDLR-LA3
| Entire | Name: Semliki Forest virus VLP in complex with the receptor VLDLR-LA3 |
|---|---|
| Components |
|
-Supramolecule #1: Semliki Forest virus VLP in complex with the receptor VLDLR-LA3
| Supramolecule | Name: Semliki Forest virus VLP in complex with the receptor VLDLR-LA3 type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:   Semliki Forest virus Semliki Forest virus |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.7 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.1 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 29408 |
|---|---|
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)