[English] 日本語
 Yorodumi
Yorodumi- EMDB-35301: Structure of mammalian spectrin-actin junctional complex of membr... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of mammalian spectrin-actin junctional complex of membrane skeleton, State I, Global map | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Macrocomplex / membrane skeleton / spectrin-actin junction / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationNCAM signaling for neurite out-growth / regulation of cellular component size / RAF/MAP kinase cascade / pointed-end actin filament capping / endoplasmic reticulum tubular network organization / negative regulation of protein targeting to membrane / regulation of multicellular organismal development / positive regulation of developmental process / spectrin / Regulation of actin dynamics for phagocytic cup formation ...NCAM signaling for neurite out-growth / regulation of cellular component size / RAF/MAP kinase cascade / pointed-end actin filament capping / endoplasmic reticulum tubular network organization / negative regulation of protein targeting to membrane / regulation of multicellular organismal development / positive regulation of developmental process / spectrin / Regulation of actin dynamics for phagocytic cup formation / EPHB-mediated forward signaling / Adherens junctions interactions / VEGFA-VEGFR2 Pathway / Cell-extracellular matrix interactions / RHO GTPases Activate WASPs and WAVEs / MAP2K and MAPK activation / lymphocyte homeostasis / UCH proteinases / lens fiber cell development / Gap junction degradation / Formation of annular gap junctions / RHOF GTPase cycle / Clathrin-mediated endocytosis / Formation of the dystrophin-glycoprotein complex (DGC) / spectrin-associated cytoskeleton / porphyrin-containing compound biosynthetic process / negative regulation of substrate adhesion-dependent cell spreading / cuticular plate / myofibril assembly / smooth endoplasmic reticulum calcium ion homeostasis / positive regulation of cellular component organization / platelet dense tubular network membrane / plasma membrane organization / negative regulation of focal adhesion assembly / regulation of filopodium assembly / cell projection membrane / cellular response to cytochalasin B / regulation of transepithelial transport / morphogenesis of a polarized epithelium / COP9 signalosome / structural constituent of postsynaptic actin cytoskeleton / protein localization to adherens junction / dense body / actin filament capping / Tat protein binding / postsynaptic actin cytoskeleton / regulation of lamellipodium assembly / adherens junction assembly / apical protein localization / RHO GTPases activate IQGAPs / RHO GTPases Activate Formins / positive regulation of fibroblast migration / tight junction / ankyrin binding / COPI-mediated anterograde transport / apical junction complex / positive regulation of wound healing / cortical actin cytoskeleton / spectrin binding / tropomyosin binding / regulation of norepinephrine uptake / erythrocyte development / nitric-oxide synthase binding / transporter regulator activity / cortical cytoskeleton / myofibril / establishment or maintenance of cell polarity / NuA4 histone acetyltransferase complex / smooth endoplasmic reticulum / brush border / hemopoiesis / striated muscle thin filament / kinesin binding / regulation of synaptic vesicle endocytosis / regulation of protein localization to plasma membrane / positive regulation of double-strand break repair via homologous recombination / positive regulation of T cell proliferation / cytoskeleton organization / axonogenesis / actin filament organization / muscle contraction / cellular response to cAMP / calyx of Held / nitric-oxide synthase regulator activity / adult locomotory behavior / cell projection / adherens junction / actin filament / cell motility / synapse organization / structural constituent of cytoskeleton / SH3 domain binding / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / Schaffer collateral - CA1 synapse / cytoplasmic ribonucleoprotein granule / actin filament binding / cell junction / nucleosome / regulation of cell shape / actin cytoskeleton Similarity search - Function | |||||||||
| Biological species |  | |||||||||
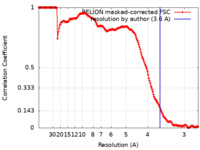
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Li N / Chen S / Gao N | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2023 Journal: Cell / Year: 2023Title: Structural basis of membrane skeleton organization in red blood cells. Authors: Ningning Li / Siyi Chen / Kui Xu / Meng-Ting He / Meng-Qiu Dong / Qiangfeng Cliff Zhang / Ning Gao /  Abstract: The spectrin-based membrane skeleton is a ubiquitous membrane-associated two-dimensional cytoskeleton underneath the lipid membrane of metazoan cells. Mutations of skeleton proteins impair the ...The spectrin-based membrane skeleton is a ubiquitous membrane-associated two-dimensional cytoskeleton underneath the lipid membrane of metazoan cells. Mutations of skeleton proteins impair the mechanical strength and functions of the membrane, leading to several different types of human diseases. Here, we report the cryo-EM structures of the native spectrin-actin junctional complex (from porcine erythrocytes), which is a specialized short F-actin acting as the central organizational unit of the membrane skeleton. While an α-/β-adducin hetero-tetramer binds to the barbed end of F-actin as a flexible cap, tropomodulin and SH3BGRL2 together create an absolute cap at the pointed end. The junctional complex is strengthened by ring-like structures of dematin in the middle actin layers and by patterned periodic interactions with tropomyosin over its entire length. This work serves as a structural framework for understanding the assembly and dynamics of membrane skeleton and offers insights into mechanisms of various ubiquitous F-actin-binding factors in other F-actin systems. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35301.map.gz emd_35301.map.gz | 398.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35301-v30.xml emd-35301-v30.xml emd-35301.xml emd-35301.xml | 31.4 KB 31.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_35301_fsc.xml emd_35301_fsc.xml | 16.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_35301.png emd_35301.png | 36.7 KB | ||
| Filedesc metadata |  emd-35301.cif.gz emd-35301.cif.gz | 11 KB | ||
| Others |  emd_35301_half_map_1.map.gz emd_35301_half_map_1.map.gz emd_35301_half_map_2.map.gz emd_35301_half_map_2.map.gz | 392 MB 392 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35301 http://ftp.pdbj.org/pub/emdb/structures/EMD-35301 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35301 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35301 | HTTPS FTP |
-Validation report
| Summary document |  emd_35301_validation.pdf.gz emd_35301_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_35301_full_validation.pdf.gz emd_35301_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_35301_validation.xml.gz emd_35301_validation.xml.gz | 24.5 KB | Display | |
| Data in CIF |  emd_35301_validation.cif.gz emd_35301_validation.cif.gz | 33.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35301 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35301 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35301 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35301 | HTTPS FTP |
-Related structure data
| Related structure data |  8iahMC  8iaiC  8ib2C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35301.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35301.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.37 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_35301_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_35301_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Spectrin-actin junctional complex
+Supramolecule #1: Spectrin-actin junctional complex
+Macromolecule #1: Adducin 1
+Macromolecule #2: Beta-adducin
+Macromolecule #3: Dematin actin binding protein
+Macromolecule #4: Spectrin alpha, erythrocytic 1
+Macromolecule #5: Actin, cytoplasmic 1
+Macromolecule #6: Spectrin beta chain
+Macromolecule #7: Tropomyosin-1.9
+Macromolecule #8: Tropomyosin 3
+Macromolecule #9: Tropomodulin-1
+Macromolecule #10: SH3 domain-binding glutamic acid-rich-like protein
+Macromolecule #11: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 34.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 2.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)