+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the SIN3S complex from S. pombe | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SIN3 / SIN3S / Pst2 / Clr6 / deacetylase / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationhistone H4K16 deacetylase activity, hydrolytic mechanism / histone H4K5 deacetylase activity, hydrolytic mechanism / histone H4K8 deacetylase activity, hydrolytic mechanism / histone H3K14 deacetylase activity, hydrolytic mechanism / HDACs deacetylate histones / histone H4K12 deacetylase activity, hydrolytic mechanism / SUMOylation of chromatin organization proteins / H3-H4 histone complex chaperone activity / histone H3K9 deacetylase activity, hydrolytic mechanism / Rpd3L complex ...histone H4K16 deacetylase activity, hydrolytic mechanism / histone H4K5 deacetylase activity, hydrolytic mechanism / histone H4K8 deacetylase activity, hydrolytic mechanism / histone H3K14 deacetylase activity, hydrolytic mechanism / HDACs deacetylate histones / histone H4K12 deacetylase activity, hydrolytic mechanism / SUMOylation of chromatin organization proteins / H3-H4 histone complex chaperone activity / histone H3K9 deacetylase activity, hydrolytic mechanism / Rpd3L complex / Rpd3L-Expanded complex / Rpd3S complex / histone deacetylase / protein lysine deacetylase activity / histone deacetylase activity / DNA repair-dependent chromatin remodeling / Sin3-type complex / NuA4 histone acetyltransferase complex / transcription initiation-coupled chromatin remodeling / epigenetic regulation of gene expression / mitotic spindle / transcription corepressor activity / heterochromatin formation / histone binding / molecular adaptor activity / chromatin remodeling / DNA repair / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / chromatin / negative regulation of transcription by RNA polymerase II / zinc ion binding / nucleus / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Wang C / Guo Z / Zhan X | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Discov / Year: 2023 Journal: Cell Discov / Year: 2023Title: Two assembly modes for SIN3 histone deacetylase complexes. Authors: Chengcheng Wang / Zhouyan Guo / Chen Chu / Yichen Lu / Xiaofeng Zhang / Xiechao Zhan /  Abstract: The switch-independent 3 (SIN3)/histone deacetylase (HDAC) complexes play essential roles in regulating chromatin accessibility and gene expression. There are two major types of SIN3/HDAC complexes ...The switch-independent 3 (SIN3)/histone deacetylase (HDAC) complexes play essential roles in regulating chromatin accessibility and gene expression. There are two major types of SIN3/HDAC complexes (named SIN3L and SIN3S) targeting different chromatin regions. Here we present the cryo-electron microscopy structures of the SIN3L and SIN3S complexes from Schizosaccharomyces pombe (S. pombe), revealing two distinct assembly modes. In the structure of SIN3L, each Sin3 isoform (Pst1 and Pst3) interacts with one histone deacetylase Clr6, and one WD40-containing protein Prw1, forming two lobes. These two lobes are bridged by two vertical coiled-coil domains from Sds3/Dep1 and Rxt2/Png2, respectively. In the structure of SIN3S, there is only one lobe organized by another Sin3 isoform Pst2; each of the Cph1 and Cph2 binds to an Eaf3 molecule, providing two modules for histone recognition and binding. Notably, the Pst1 Lobe in SIN3L and the Pst2 Lobe in SIN3S adopt similar conformation with their deacetylase active sites exposed to the space; however, the Pst3 Lobe in SIN3L is in a compact state with its active center buried inside and blocked. Our work reveals two classical organization mechanisms for the SIN3/HDAC complexes to achieve specific targeting and provides a framework for studying the histone deacetylase complexes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35092.map.gz emd_35092.map.gz | 167.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35092-v30.xml emd-35092-v30.xml emd-35092.xml emd-35092.xml | 23.2 KB 23.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35092.png emd_35092.png | 63.6 KB | ||
| Filedesc metadata |  emd-35092.cif.gz emd-35092.cif.gz | 8 KB | ||
| Others |  emd_35092_half_map_1.map.gz emd_35092_half_map_1.map.gz emd_35092_half_map_2.map.gz emd_35092_half_map_2.map.gz | 165.1 MB 165.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35092 http://ftp.pdbj.org/pub/emdb/structures/EMD-35092 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35092 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35092 | HTTPS FTP |
-Related structure data
| Related structure data |  8i02MC  8i03C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_35092.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35092.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.087 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_35092_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
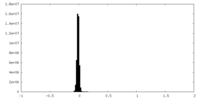
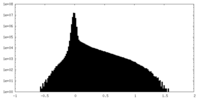
| Density Histograms |
-Half map: #1
| File | emd_35092_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
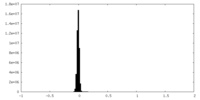
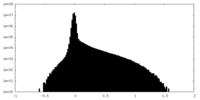
| Density Histograms |
- Sample components
Sample components
-Entire : The SIN3S complex
| Entire | Name: The SIN3S complex |
|---|---|
| Components |
|
-Supramolecule #1: The SIN3S complex
| Supramolecule | Name: The SIN3S complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#6 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Cph1
| Macromolecule | Name: Cph1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 45.046082 KDa |
| Sequence | String: MASSINNSSQ PTVPSISNNS HGDSFVNEGP PSNFKNNSLT SSTHSSTDHV NVLPISQDKE MDISSPVKKQ KASYSNKSPN KAPIQKSRG SSLKSHLETE SQQTPVKRRR RKATIRNVDY CSACGGRGLF ICCEGCPCSF HLSCLEPPLT PENIPEGSWF C VTCSIKSH ...String: MASSINNSSQ PTVPSISNNS HGDSFVNEGP PSNFKNNSLT SSTHSSTDHV NVLPISQDKE MDISSPVKKQ KASYSNKSPN KAPIQKSRG SSLKSHLETE SQQTPVKRRR RKATIRNVDY CSACGGRGLF ICCEGCPCSF HLSCLEPPLT PENIPEGSWF C VTCSIKSH HPPKHPLSIW SQLYDWIDSQ NPSQYRLPDD LVHYFHGISR GDTGAYKETE GEMDTDEFSA LPTGSSITNL AY CGYCSKP SMGACWVYGC QLCDTFYHKN CKEHAKKCSH DSIGKKGMRV PKNAVVIRTP LVLDTTSNTL NPKVMISGWQ FLM GEFPSD ELLYFPRLPV SCLYKVSEDG LIKDFLYAIG IEAKKFNNER KKRELEVIPP DVKSALLPAR THPNLPIALR TLFN KART UniProtKB: Uncharacterized protein C16C9.05 |
-Macromolecule #2: Paired amphipathic helix protein pst2
| Macromolecule | Name: Paired amphipathic helix protein pst2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 125.011609 KDa |
| Sequence | String: MEQTLAILKN DNSTLVAEMQ NQLVHDFSPN GTALPELDIK AFVQKLGQRL CHRPYVYSAF MDVVKALHNE IVDFPGFIER ISVILRDYP DLLEYLNIFL PSSYKYLLSN SGANFTLQFT TPSGPVSTPS TYVATYNDLP CTYHRAIGFV SRVRRALLSN P EQFFKLQD ...String: MEQTLAILKN DNSTLVAEMQ NQLVHDFSPN GTALPELDIK AFVQKLGQRL CHRPYVYSAF MDVVKALHNE IVDFPGFIER ISVILRDYP DLLEYLNIFL PSSYKYLLSN SGANFTLQFT TPSGPVSTPS TYVATYNDLP CTYHRAIGFV SRVRRALLSN P EQFFKLQD SLRKFKNSEC SLSELQTIVT SLLAEHPSLA HEFHNFLPSS IFFGSKPPLG SFPLRGIQSS QFTLSNISDL LS QSRPDNL SPFSHLSNES SDFFKNVKNV LTDVETYHEF LKLLNLYVQG IIDRNILVSR GFGFLKSNSG LWRSFLSLTS LSP EEFLSV YNSACSDFPE CGPSYRLLPV EERNISCSGR DDFAWGILND DWVSHPTWAS EESGFIVQRK TPYEEAMTKL EEER YEFDR HIEATSWTIK SLKKIQNRIN ELPEEERETY TLEEGLGLPS KSIYKKTIKL VYTSEHAEEM FKALERMPCL TLPLV ISRL EEKNEEWKSV KRSLQPGWRS IEFKNYDKSL DSQCVYFKAR DKKNVSSKFL LAEADILRSQ AKLHFPLRSR SAFEFS FVY DNEIVLFDTC YMVCTYIVCN SPSGLKKVEH FFKNILPLHF GLEKDKFSIF LDQVFRGPDY DVNAPNIVGN KPVRRKR SN SITQLTEFVK QPKINGQRES RSAAAARKKE ESGNKSQSNS QNSLSDESGN VTPVSKKQLS QPAAAIKASL KYPSHPDS L LEHQDHAGDT ENEMHDDVDK EQFGYSSMYV FFRLFNLLYE RLYELQRLED QVSIIQQRII PNPVSQKQKI WRDRWNDLS DVPDEKTHYE NTYVMILRLI YGIVDQSAFE DYLRFYYGNK AYKIYTIDKL VWSAAKQVHH IVSDGKYKFV TSLVEQNSSA SPKKNYDDF LYRLEIEKLL NPDEILFRFC WINKFKSFGI KIMKRANLIV DQSLDTQRRV WKKYVQNYRI QKLTEEISYK N YRCPFLCR NIEKERTVEQ LVSRLQTKLL RSAELVSGLQ AKLCLDSFKL LYLPRTEDSY IDASYLRLRD TDFLDCQNKR KQ RWRNRWE SLLKSVRGTS DNTAEVNFDA DINALFIP UniProtKB: Paired amphipathic helix protein pst2 |
-Macromolecule #3: Histone deacetylase clr6
| Macromolecule | Name: Histone deacetylase clr6 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: histone deacetylase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 46.165844 KDa |
| Sequence | String: MGFGKKKVSY FYDEDVGNYH YGPQHPMKPH RVRMVHNLVV NYNLYEKLNV ITPVRATRND MTRCHTDEYI EFLWRVTPDT MEKFQPHQL KFNVGDDCPV FDGLYEFCSI SAGGSIGAAQ ELNSGNAEIA INWAGGLHHA KKREASGFCY VNDIALAALE L LKYHQRVL ...String: MGFGKKKVSY FYDEDVGNYH YGPQHPMKPH RVRMVHNLVV NYNLYEKLNV ITPVRATRND MTRCHTDEYI EFLWRVTPDT MEKFQPHQL KFNVGDDCPV FDGLYEFCSI SAGGSIGAAQ ELNSGNAEIA INWAGGLHHA KKREASGFCY VNDIALAALE L LKYHQRVL YIDIDVHHGD GVEEFFYTTD RVMTCSFHKF GEYFPGTGHI KDTGIGTGKN YAVNVPLRDG IDDESYESVF KP VISHIMQ WFRPEAVILQ CGTDSLAGDR LGCFNLSMKG HSMCVDFVKS FNLPMICVGG GGYTVRNVAR VWTYETGLLA GEE LDENLP YNDYLQYYGP DYKLNVLSNN MENHNTRQYL DSITSEIIEN LRNLSFAPSV QMHKTPGDFT FENAEKQNIA KEEI MDERV UniProtKB: Histone deacetylase clr6 |
-Macromolecule #4: RbAp48-related WD40 repeat-containing protein prw1
| Macromolecule | Name: RbAp48-related WD40 repeat-containing protein prw1 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 48.528926 KDa |
| Sequence | String: MAVSAVPHPS KQAQASEEGI NQEKCINEEY KIWKKNSPFL YDLIITRALE WPCMSLQWYP EQQIFAEHGY TEQKMFLGVR ADVGKYLLA VASIQLPYLN QTVPPTTMEG ASAGDESSLR VNISNLYSHP ESVCSAKLMP QDDSCVATVG NYHNDVLVFD K ESFESYSS ...String: MAVSAVPHPS KQAQASEEGI NQEKCINEEY KIWKKNSPFL YDLIITRALE WPCMSLQWYP EQQIFAEHGY TEQKMFLGVR ADVGKYLLA VASIQLPYLN QTVPPTTMEG ASAGDESSLR VNISNLYSHP ESVCSAKLMP QDDSCVATVG NYHNDVLVFD K ESFESYSS ASESPLKPKY RLTKHTQPCT SVCWNFLSKG TLVSGSQDAT LSCWDLNAYN ESDSASVLKV HISSHEKQVS DV RFHYKHQ DLLASVSYDQ YLHVHDIRRP DASTKPARSV HAHSGPIHSV AFNPHNDFIL ATCSTDKTIA LWDLRNLNQR LHT LEGHED IVTKISFSPH EEPILASTSA DRRTLVWDLS RIGEDQPAEE AQDGPPELLF MHGGHTSCTI DMDWCPNYNW TMAT AAEDN ILQIWTPSRS IWGNEQLEED ATAYLS UniProtKB: RbAp48-related WD40 repeat-containing protein prw1 |
-Macromolecule #5: Chromatin modification-related protein eaf3
| Macromolecule | Name: Chromatin modification-related protein eaf3 / type: protein_or_peptide / ID: 5 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 39.191199 KDa |
| Sequence | String: MAVSYKVNER VLCFHGPLLY EAKIVDTEMK GDVTTYLIHY KGWKNSWDEW VEQDRILQWT EENLKTQKEL KNAAISTRQK PTSKKSASS TSKHDSTGVK TSGKRSRESS TVTVDGDSHE LPSRIKTQKS ESPIPQQVKR DGTTDAKNEE TTKPENNEKD D FEEEPPLP ...String: MAVSYKVNER VLCFHGPLLY EAKIVDTEMK GDVTTYLIHY KGWKNSWDEW VEQDRILQWT EENLKTQKEL KNAAISTRQK PTSKKSASS TSKHDSTGVK TSGKRSRESS TVTVDGDSHE LPSRIKTQKS ESPIPQQVKR DGTTDAKNEE TTKPENNEKD D FEEEPPLP KHKISVPDVL KLWLVDDWEN ITKNQQLIAI PRNPTVRAAI AAFRESKISH LNNEIDVDVF EQAMAGLVIY FN KCLGNML LYRFERQQYL EIRQQYPDTE MCDLYGVEHL IRLFVSLPEL IDRTNMDSQS IECLLNYIEE FLKYLVLHKD EYF IKEYQN APPNYRSLVG V UniProtKB: Chromatin modification-related protein eaf3 |
-Macromolecule #6: Uncharacterized protein C2F7.07c
| Macromolecule | Name: Uncharacterized protein C2F7.07c / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 68.871703 KDa |
| Sequence | String: MDAKPWNHTS EAFQASILED LKIIQKAGAE RNAKSSHGSI NSRSASPNKA TSRRNRAQNG NSNGRASVDN SDDGSKDDLD YSPSVKRKH VNGEGAEKGD HDTSNNGPSI TKLRRKVRRT YDTKDGFVAW NTLDDDFRPI VPDQERSRKI NPQKGNNNNL L KENKSLKT ...String: MDAKPWNHTS EAFQASILED LKIIQKAGAE RNAKSSHGSI NSRSASPNKA TSRRNRAQNG NSNGRASVDN SDDGSKDDLD YSPSVKRKH VNGEGAEKGD HDTSNNGPSI TKLRRKVRRT YDTKDGFVAW NTLDDDFRPI VPDQERSRKI NPQKGNNNNL L KENKSLKT TAKDLSDISS SSMKKANNSS KPLFSGKLTF KANIPVPTSE VVTENNVTRN VTVYSNQKHL GNESENFNDM EG RAEDISS NELLPTPEEY PYRYNNDYCS ACHGPGNFLC CETCPNSFHF TCIDPPIEEK NLPDDAWYCN ECKHHSLYNE LDE QEELES NVKEEGTMVD VWMQLCTYID SHNPIQFHLP HSISSFFRGV GSGVMGEYIE TDVLKHLKSS RRSNGEERDP LLLK SKSGT PILCFRCHKS ALVSQSILAC DYCNSYWHPD CLNPPLATLP SNLRKWKCPN HSDHVTPRYR LPEKAKVIRV GLPRG FKNK GNIVIDENED EPSVQTIQLQ GKIRVVPSKP FKLNFLEQIR DNVINLRKMV EQDEQLCIET FSKFDFYATR DCELPL RIL CDVANDNLEN DDYVLALRDL LRISKWDPNQ PVPAPFDLAN LLSY UniProtKB: Uncharacterized protein C2F7.07c |
-Macromolecule #7: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 7 / Number of copies: 6 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #8: POTASSIUM ION
| Macromolecule | Name: POTASSIUM ION / type: ligand / ID: 8 / Number of copies: 2 / Formula: K |
|---|---|
| Molecular weight | Theoretical: 39.098 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.3000000000000003 µm / Nominal defocus min: 1.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)