[English] 日本語
 Yorodumi
Yorodumi- EMDB-34655: SARS-CoV-2 Omicron BA.1 spike trimer (6P) in complex with YB13-29... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | SARS-CoV-2 Omicron BA.1 spike trimer (6P) in complex with YB13-292 Fab, focused refinement of Fab region | ||||||||||||
 Map data Map data | Focused half map 2 of YB13-292 Fab complexed with Omicron S | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Spike protein / RBD / antibody / Fab / Viral protein / VIRAL PROTEIN-IMMUNE SYSTEM complex | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion ...symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion / entry receptor-mediated virion attachment to host cell / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||||||||
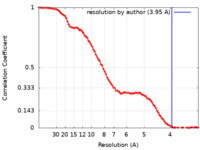
| Method | single particle reconstruction / cryo EM / Resolution: 3.95 Å | ||||||||||||
 Authors Authors | Liu B / Gao X / Chen Q / Li Z / Su M / He J / Xiong X | ||||||||||||
| Funding support | 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Somatically hypermutated antibodies isolated from SARS-CoV-2 Delta infected patients cross-neutralize heterologous variants. Authors: Haisheng Yu / Banghui Liu / Yudi Zhang / Xijie Gao / Qian Wang / Haitao Xiang / Xiaofang Peng / Caixia Xie / Yaping Wang / Peiyu Hu / Jingrong Shi / Quan Shi / Pingqian Zheng / Chengqian ...Authors: Haisheng Yu / Banghui Liu / Yudi Zhang / Xijie Gao / Qian Wang / Haitao Xiang / Xiaofang Peng / Caixia Xie / Yaping Wang / Peiyu Hu / Jingrong Shi / Quan Shi / Pingqian Zheng / Chengqian Feng / Guofang Tang / Xiaopan Liu / Liliangzi Guo / Xiumei Lin / Jiaojiao Li / Chuanyu Liu / Yaling Huang / Naibo Yang / Qiuluan Chen / Zimu Li / Mengzhen Su / Qihong Yan / Rongjuan Pei / Xinwen Chen / Longqi Liu / Fengyu Hu / Dan Liang / Bixia Ke / Changwen Ke / Feng Li / Jun He / Meiniang Wang / Ling Chen / Xiaoli Xiong / Xiaoping Tang /  Abstract: SARS-CoV-2 Omicron variants feature highly mutated spike proteins with extraordinary abilities in evading antibodies isolated earlier in the pandemic. Investigation of memory B cells from patients ...SARS-CoV-2 Omicron variants feature highly mutated spike proteins with extraordinary abilities in evading antibodies isolated earlier in the pandemic. Investigation of memory B cells from patients primarily with breakthrough infections with the Delta variant enables isolation of a number of neutralizing antibodies cross-reactive to heterologous variants of concern (VOCs) including Omicron variants (BA.1-BA.4). Structural studies identify altered complementarity determining region (CDR) amino acids and highly unusual heavy chain CDR2 insertions respectively in two representative cross-neutralizing antibodies-YB9-258 and YB13-292. These features are putatively introduced by somatic hypermutation and they are heavily involved in epitope recognition to broaden neutralization breadth. Previously, insertions/deletions were rarely reported for antiviral antibodies except for those induced by HIV-1 chronic infections. These data provide molecular mechanisms for cross-neutralization of heterologous SARS-CoV-2 variants by antibodies isolated from Delta variant infected patients with implications for future vaccination strategy. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34655.map.gz emd_34655.map.gz | 59.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34655-v30.xml emd-34655-v30.xml emd-34655.xml emd-34655.xml | 24.4 KB 24.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_34655_fsc.xml emd_34655_fsc.xml | 11.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_34655.png emd_34655.png | 26.1 KB | ||
| Filedesc metadata |  emd-34655.cif.gz emd-34655.cif.gz | 7.1 KB | ||
| Others |  emd_34655_half_map_1.map.gz emd_34655_half_map_1.map.gz emd_34655_half_map_2.map.gz emd_34655_half_map_2.map.gz | 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34655 http://ftp.pdbj.org/pub/emdb/structures/EMD-34655 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34655 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34655 | HTTPS FTP |
-Validation report
| Summary document |  emd_34655_validation.pdf.gz emd_34655_validation.pdf.gz | 798.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34655_full_validation.pdf.gz emd_34655_full_validation.pdf.gz | 798 KB | Display | |
| Data in XML |  emd_34655_validation.xml.gz emd_34655_validation.xml.gz | 15.5 KB | Display | |
| Data in CIF |  emd_34655_validation.cif.gz emd_34655_validation.cif.gz | 21 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34655 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34655 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34655 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34655 | HTTPS FTP |
-Related structure data
| Related structure data |  8hc8MC  8hc2C  8hc3C  8hc4C  8hc5C  8hc6C  8hc7C  8hc9C  8hcaC  8hcbC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34655.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34655.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused half map 2 of YB13-292 Fab complexed with Omicron S | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.65 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Focused map of YB13-292 Fab complexed with Omicron S
| File | emd_34655_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused map of YB13-292 Fab complexed with Omicron S | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Focused half map 1 of YB13-292 Fab complexed with Omicron S
| File | emd_34655_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused half map 1 of YB13-292 Fab complexed with Omicron S | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : SARS-CoV-2 Omicron BA.1 spike trimer with in complex with YB13-29...
| Entire | Name: SARS-CoV-2 Omicron BA.1 spike trimer with in complex with YB13-292 Fab, focused refinement of Fab region |
|---|---|
| Components |
|
-Supramolecule #1: SARS-CoV-2 Omicron BA.1 spike trimer with in complex with YB13-29...
| Supramolecule | Name: SARS-CoV-2 Omicron BA.1 spike trimer with in complex with YB13-292 Fab, focused refinement of Fab region type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #2: SARS-CoV-2 Omicron BA.1 spike protein
| Supramolecule | Name: SARS-CoV-2 Omicron BA.1 spike protein / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: YB13-292 Fab
| Supramolecule | Name: YB13-292 Fab / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Spike protein S1
| Macromolecule | Name: Spike protein S1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 21.33207 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: ITNLCPFDEV FNATRFASVY AWNRKRISNC VADYSVLYNL APFFTFKCYG VSPTKLNDLC FTNVYADSFV IRGDEVRQIA PGQTGNIAD YNYKLPDDFT GCVIAWNSNK LDSKVSGNYN YLYRLFRKSN LKPFERDIST EIYQAGNKPC NGVAGFNCYF P LRSYSFRP TYGVGHQPYR VVVLSFEL UniProtKB: Spike glycoprotein |
-Macromolecule #2: Heavy chain of YB13-292 Fab
| Macromolecule | Name: Heavy chain of YB13-292 Fab / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 25.283396 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EVQLVESGGG LVKPGGSLRL SCAASGFSFI TYNMNWVRQA PGKGLEWVSS ISSNILSSTS YIYYADSVKG RFTISRDDAA NSLFLQMNS LRVEDTAQYY CARTRSRSVR NCTSATCPVD AFDLWGQGTM VIVSSASTKG PSVFPLAPSS KSTSGGTAAL G CLVKDYFP ...String: EVQLVESGGG LVKPGGSLRL SCAASGFSFI TYNMNWVRQA PGKGLEWVSS ISSNILSSTS YIYYADSVKG RFTISRDDAA NSLFLQMNS LRVEDTAQYY CARTRSRSVR NCTSATCPVD AFDLWGQGTM VIVSSASTKG PSVFPLAPSS KSTSGGTAAL G CLVKDYFP EPVTVSWNSG ALTSGVHTFP AVLQSSGLYS LSSVVTVPSS SLGTQTYICN VNHKPSNTKV DKKVEPKSCD |
-Macromolecule #3: Light chain of YB13-292 Fab
| Macromolecule | Name: Light chain of YB13-292 Fab / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.976648 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DIVLTQSPLS LPVTPGEPAS ISCRSSQSLL RSNGYNYLDW YLQKPGQSPH LLIYLGSNRA SGVPDRFSGS GSGTDFTLKI SRVEAEDVG VYYCMQALQT PYTFGQGTNL EIKRTVAAPS VFIFPPSDEQ LKSGTASVVC LLNNFYPREA KVQWKVDNAL Q SGNSQESV ...String: DIVLTQSPLS LPVTPGEPAS ISCRSSQSLL RSNGYNYLDW YLQKPGQSPH LLIYLGSNRA SGVPDRFSGS GSGTDFTLKI SRVEAEDVG VYYCMQALQT PYTFGQGTNL EIKRTVAAPS VFIFPPSDEQ LKSGTASVVC LLNNFYPREA KVQWKVDNAL Q SGNSQESV TEQDSKDSTY SLSSTLTLSK ADYEKHKVYA CEVTHQGLSS PVTKSFNRGE C |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 1 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)