[English] 日本語
 Yorodumi
Yorodumi- EMDB-34499: Cryo-EM map of SARS-CoV-2 Omicron BA.2 spike protein in complex w... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of SARS-CoV-2 Omicron BA.2 spike protein in complex with mouse ACE2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SARS-CoV-2 / Omicron BA.2 / spike protein / VIRAL PROTEIN / HYDROLASE-VIRAL PROTEIN complex | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.02 Å | |||||||||
 Authors Authors | Zhao ZN / Xie YF / Chai Y / Qi JX / Gao GF | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis for receptor binding and broader interspecies receptor recognition of currently circulating Omicron sub-variants. Authors: Zhennan Zhao / Yufeng Xie / Bin Bai / Chunliang Luo / Jingya Zhou / Weiwei Li / Yumin Meng / Linjie Li / Dedong Li / Xiaomei Li / Xiaoxiong Li / Xiaoyun Wang / Junqing Sun / Zepeng Xu / ...Authors: Zhennan Zhao / Yufeng Xie / Bin Bai / Chunliang Luo / Jingya Zhou / Weiwei Li / Yumin Meng / Linjie Li / Dedong Li / Xiaomei Li / Xiaoxiong Li / Xiaoyun Wang / Junqing Sun / Zepeng Xu / Yeping Sun / Wei Zhang / Zheng Fan / Xin Zhao / Linhuan Wu / Juncai Ma / Odel Y Li / Guijun Shang / Yan Chai / Kefang Liu / Peiyi Wang / George F Gao / Jianxun Qi /  Abstract: Multiple SARS-CoV-2 Omicron sub-variants, such as BA.2, BA.2.12.1, BA.4, and BA.5, emerge one after another. BA.5 has become the dominant strain worldwide. Additionally, BA.2.75 is significantly ...Multiple SARS-CoV-2 Omicron sub-variants, such as BA.2, BA.2.12.1, BA.4, and BA.5, emerge one after another. BA.5 has become the dominant strain worldwide. Additionally, BA.2.75 is significantly increasing in some countries. Exploring their receptor binding and interspecies transmission risk is urgently needed. Herein, we examine the binding capacities of human and other 28 animal ACE2 orthologs covering nine orders towards S proteins of these sub-variants. The binding affinities between hACE2 and these sub-variants remain in the range as that of previous variants of concerns (VOCs) or interests (VOIs). Notably, R493Q reverse mutation enhances the bindings towards ACE2s from humans and many animals closely related to human life, suggesting an increased risk of cross-species transmission. Structures of S/hACE2 or RBD/hACE2 complexes for these sub-variants and BA.2 S binding to ACE2 of mouse, rat or golden hamster are determined to reveal the molecular basis for receptor binding and broader interspecies recognition. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34499.map.gz emd_34499.map.gz | 453.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34499-v30.xml emd-34499-v30.xml emd-34499.xml emd-34499.xml | 13.9 KB 13.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34499.png emd_34499.png | 38.1 KB | ||
| Others |  emd_34499_half_map_1.map.gz emd_34499_half_map_1.map.gz emd_34499_half_map_2.map.gz emd_34499_half_map_2.map.gz | 474.7 MB 474.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34499 http://ftp.pdbj.org/pub/emdb/structures/EMD-34499 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34499 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34499 | HTTPS FTP |
-Validation report
| Summary document |  emd_34499_validation.pdf.gz emd_34499_validation.pdf.gz | 892.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34499_full_validation.pdf.gz emd_34499_full_validation.pdf.gz | 892 KB | Display | |
| Data in XML |  emd_34499_validation.xml.gz emd_34499_validation.xml.gz | 18.8 KB | Display | |
| Data in CIF |  emd_34499_validation.cif.gz emd_34499_validation.cif.gz | 22.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34499 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34499 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34499 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34499 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_34499.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34499.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.67 Å | ||||||||||||||||||||||||||||||||||||
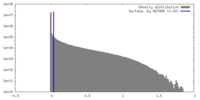
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_34499_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
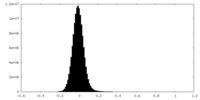
| Density Histograms |
-Half map: #2
| File | emd_34499_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
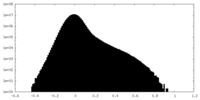
| Density Histograms |
- Sample components
Sample components
-Entire : SARS-CoV-2 Omicron BA.2 spike protein in complex with mouse ACE2
| Entire | Name: SARS-CoV-2 Omicron BA.2 spike protein in complex with mouse ACE2 |
|---|---|
| Components |
|
-Supramolecule #1: SARS-CoV-2 Omicron BA.2 spike protein in complex with mouse ACE2
| Supramolecule | Name: SARS-CoV-2 Omicron BA.2 spike protein in complex with mouse ACE2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|
-Supramolecule #2: Mouse angiotensin-converting enzyme 2
| Supramolecule | Name: Mouse angiotensin-converting enzyme 2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: Omicron BA.2 spike protein
| Supramolecule | Name: Omicron BA.2 spike protein / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.02 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 91073 |
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: OTHER |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)