[English] 日本語
 Yorodumi
Yorodumi- EMDB-34120: Cryo-EM structure of SARS-CoV-2 Omicron BA.2 RBD in complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of SARS-CoV-2 Omicron BA.2 RBD in complex with golden hamster ACE2 (local refinement) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SARS-CoV-2 / Omicron BA.2 / spike protein / VIRAL PROTEIN / HYDROLASE-VIRAL PROTEIN complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationHydrolases; Acting on peptide bonds (peptidases) / positive regulation of L-proline import across plasma membrane / angiotensin-mediated drinking behavior / positive regulation of gap junction assembly / tryptophan transport / regulation of cardiac conduction / peptidyl-dipeptidase activity / transporter activator activity / angiotensin maturation / metallocarboxypeptidase activity ...Hydrolases; Acting on peptide bonds (peptidases) / positive regulation of L-proline import across plasma membrane / angiotensin-mediated drinking behavior / positive regulation of gap junction assembly / tryptophan transport / regulation of cardiac conduction / peptidyl-dipeptidase activity / transporter activator activity / angiotensin maturation / metallocarboxypeptidase activity / positive regulation of cardiac muscle contraction / brush border membrane / virus receptor activity / regulation of inflammatory response / endopeptidase activity / symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion / entry receptor-mediated virion attachment to host cell / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / cilium / symbiont-mediated suppression of host innate immune response / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / cell surface / negative regulation of transcription by RNA polymerase II / extracellular space / metal ion binding / identical protein binding / membrane / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Mesocricetus auratus (golden hamster) / Mesocricetus auratus (golden hamster) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.94 Å | |||||||||
 Authors Authors | Zhao ZN / Xie YF / Chai Y / Qi JX / Gao GF | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis for receptor binding and broader interspecies receptor recognition of currently circulating Omicron sub-variants. Authors: Zhennan Zhao / Yufeng Xie / Bin Bai / Chunliang Luo / Jingya Zhou / Weiwei Li / Yumin Meng / Linjie Li / Dedong Li / Xiaomei Li / Xiaoxiong Li / Xiaoyun Wang / Junqing Sun / Zepeng Xu / ...Authors: Zhennan Zhao / Yufeng Xie / Bin Bai / Chunliang Luo / Jingya Zhou / Weiwei Li / Yumin Meng / Linjie Li / Dedong Li / Xiaomei Li / Xiaoxiong Li / Xiaoyun Wang / Junqing Sun / Zepeng Xu / Yeping Sun / Wei Zhang / Zheng Fan / Xin Zhao / Linhuan Wu / Juncai Ma / Odel Y Li / Guijun Shang / Yan Chai / Kefang Liu / Peiyi Wang / George F Gao / Jianxun Qi /  Abstract: Multiple SARS-CoV-2 Omicron sub-variants, such as BA.2, BA.2.12.1, BA.4, and BA.5, emerge one after another. BA.5 has become the dominant strain worldwide. Additionally, BA.2.75 is significantly ...Multiple SARS-CoV-2 Omicron sub-variants, such as BA.2, BA.2.12.1, BA.4, and BA.5, emerge one after another. BA.5 has become the dominant strain worldwide. Additionally, BA.2.75 is significantly increasing in some countries. Exploring their receptor binding and interspecies transmission risk is urgently needed. Herein, we examine the binding capacities of human and other 28 animal ACE2 orthologs covering nine orders towards S proteins of these sub-variants. The binding affinities between hACE2 and these sub-variants remain in the range as that of previous variants of concerns (VOCs) or interests (VOIs). Notably, R493Q reverse mutation enhances the bindings towards ACE2s from humans and many animals closely related to human life, suggesting an increased risk of cross-species transmission. Structures of S/hACE2 or RBD/hACE2 complexes for these sub-variants and BA.2 S binding to ACE2 of mouse, rat or golden hamster are determined to reveal the molecular basis for receptor binding and broader interspecies recognition. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34120.map.gz emd_34120.map.gz | 454.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34120-v30.xml emd-34120-v30.xml emd-34120.xml emd-34120.xml | 21.1 KB 21.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34120.png emd_34120.png | 36.5 KB | ||
| Filedesc metadata |  emd-34120.cif.gz emd-34120.cif.gz | 6.8 KB | ||
| Others |  emd_34120_half_map_1.map.gz emd_34120_half_map_1.map.gz emd_34120_half_map_2.map.gz emd_34120_half_map_2.map.gz | 474.9 MB 474.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34120 http://ftp.pdbj.org/pub/emdb/structures/EMD-34120 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34120 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34120 | HTTPS FTP |
-Validation report
| Summary document |  emd_34120_validation.pdf.gz emd_34120_validation.pdf.gz | 795.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34120_full_validation.pdf.gz emd_34120_full_validation.pdf.gz | 795 KB | Display | |
| Data in XML |  emd_34120_validation.xml.gz emd_34120_validation.xml.gz | 18.4 KB | Display | |
| Data in CIF |  emd_34120_validation.cif.gz emd_34120_validation.cif.gz | 21.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34120 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34120 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34120 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34120 | HTTPS FTP |
-Related structure data
| Related structure data |  7yv8MC  7yhwC  7yj3C  7yvuC  8gryC  8h06C  8h5cC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34120.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34120.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.88 Å | ||||||||||||||||||||||||||||||||||||
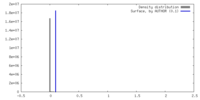
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34120_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
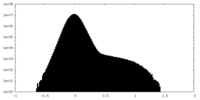
| Density Histograms |
-Half map: #1
| File | emd_34120_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of SARS-CoV-2 Omicron BA.2 RBD in complex with ...
| Entire | Name: Cryo-EM structure of SARS-CoV-2 Omicron BA.2 RBD in complex with golden hamster ACE2 (local refinement) |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of SARS-CoV-2 Omicron BA.2 RBD in complex with ...
| Supramolecule | Name: Cryo-EM structure of SARS-CoV-2 Omicron BA.2 RBD in complex with golden hamster ACE2 (local refinement) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|
-Supramolecule #2: Golden hamster angiotensin-converting enzyme 2
| Supramolecule | Name: Golden hamster angiotensin-converting enzyme 2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Mesocricetus auratus (golden hamster) Mesocricetus auratus (golden hamster) |
-Supramolecule #3: Omicron BA.2 RBD
| Supramolecule | Name: Omicron BA.2 RBD / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Angiotensin-converting enzyme
| Macromolecule | Name: Angiotensin-converting enzyme / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: Hydrolases; Acting on peptide bonds (peptidases) |
|---|---|
| Source (natural) | Organism:  Mesocricetus auratus (golden hamster) Mesocricetus auratus (golden hamster) |
| Molecular weight | Theoretical: 68.943328 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: SIIEEQAKTF LDKFNQEAED LSYQSALASW NYNTNITEEN AQKMNEAAAK WSAFYEEQSK LAKNYSLQEV QNLTIKRQLQ ALQQSGSSA LSADKNKQLN TILNTMSTIY STGKVCNPKN PQECLLLEPG LDDIMATSTD YNERLWAWEG WRAEVGKQLR P LYEEYVVL ...String: SIIEEQAKTF LDKFNQEAED LSYQSALASW NYNTNITEEN AQKMNEAAAK WSAFYEEQSK LAKNYSLQEV QNLTIKRQLQ ALQQSGSSA LSADKNKQLN TILNTMSTIY STGKVCNPKN PQECLLLEPG LDDIMATSTD YNERLWAWEG WRAEVGKQLR P LYEEYVVL KNEMARANNY EDYGDYWRGD YEAEGADGYN YNGNQLIEDV ERTFKEIKPL YEQLHAYVRT KLMNTYPSYI SP TGCLPAH LLGDMWGRFW TNLYPLTVPF GQKPNIDVTD AMVNQGWNAE RIFKEAEKFF VSVGLPYMTQ GFWENSMLTD PGD DRKVVC HPTAWDLGKG DFRIKMCTKV TMDNFLTAHH EMGHIQYDMA YATQPFLLRN GANEGFHEAV GEIMSLSAAT PEHL KSIGL LPSDFQEDNE TEINFLLKQA LTIVGTLPFT YMLEKWRWMV FKGDIPKEQW MEKWWEMKRE IVGVVEPLPH DETYC DPAA LFHVSNDYSF IRYYTRTIYQ FQFQEALCQA AKHDGPLHKC DISNSTEAGQ KLLNMLRLGK SEPWTLALEN VVGARN MDV RPLLNYFEPL SVWLKEQNKN SFVGWNTDWS PYA UniProtKB: Angiotensin-converting enzyme |
-Macromolecule #2: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 2 / Details: Omicron BA.2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 22.282211 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: ITNLCPFDEV FNATRFASVY AWNRKRISNC VADYSVLYNF APFFAFKCYG VSPTKLNDLC FTNVYADSFV IRGNEVSQIA PGQTGNIAD YNYKLPDDFT GCVIAWNSNK LDSKVGGNYN YLYRLFRKSN LKPFERDIST EIYQAGNKPC NGVAGFNCYF P LRSYGFRP ...String: ITNLCPFDEV FNATRFASVY AWNRKRISNC VADYSVLYNF APFFAFKCYG VSPTKLNDLC FTNVYADSFV IRGNEVSQIA PGQTGNIAD YNYKLPDDFT GCVIAWNSNK LDSKVGGNYN YLYRLFRKSN LKPFERDIST EIYQAGNKPC NGVAGFNCYF P LRSYGFRP TYGVGHQPYR VVVLSFELLH APATVCGPK UniProtKB: Spike glycoprotein |
-Macromolecule #3: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 3 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 6 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)