+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of DHEA-ADGRG2-FL-Gs complex | |||||||||||||||
 Map data Map data | The cryo-em density of DHEA-ADGRG2-FL-Gs complex | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | ADGRG2 / GPCR / MEMBRANE PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationspermatid development / G protein-coupled receptor activity / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels ...spermatid development / G protein-coupled receptor activity / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through CDC42 / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / cellular response to catecholamine stimulus / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / adenylate cyclase-activating dopamine receptor signaling pathway / GPER1 signaling / Inactivation, recovery and regulation of the phototransduction cascade / cellular response to prostaglandin E stimulus / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / sensory perception of taste / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Ras protein signal transduction / Extra-nuclear estrogen signaling / cell population proliferation / cell surface receptor signaling pathway / G protein-coupled receptor signaling pathway / apical plasma membrane / lysosomal membrane / GTPase activity / synapse / protein-containing complex binding / signal transduction / extracellular exosome / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /  | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||||||||
 Authors Authors | Guo SC / Xiao P / Lin H / Sun JP / Yu X | |||||||||||||||
| Funding support |  China, 4 items China, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2022 Journal: Nat Chem Biol / Year: 2022Title: Structures of the ADGRG2-G complex in apo and ligand-bound forms. Authors: Hui Lin / Peng Xiao / Rui-Qian Bu / Shengchao Guo / Zhao Yang / Daopeng Yuan / Zhong-Liang Zhu / Chuan-Xin Zhang / Qing-Tao He / Chao Zhang / Yu-Qi Ping / Ru-Jia Zhao / Chuan-Shun Ma / Chang- ...Authors: Hui Lin / Peng Xiao / Rui-Qian Bu / Shengchao Guo / Zhao Yang / Daopeng Yuan / Zhong-Liang Zhu / Chuan-Xin Zhang / Qing-Tao He / Chao Zhang / Yu-Qi Ping / Ru-Jia Zhao / Chuan-Shun Ma / Chang-Hao Liu / Xiao-Ning Zhang / Dan Jiang / Shaohui Huang / Yue-Tong Xi / Dao-Lai Zhang / Chen-Yang Xue / Bai-Sheng Yang / Jian-Yuan Li / Hao-Cheng Lin / Xu-Hui Zeng / Han Zhao / Wen-Ming Xu / Fan Yi / Zhongmin Liu / Jin-Peng Sun / Xiao Yu /  Abstract: Adhesion G protein-coupled receptors are elusive in terms of their structural information and ligands. Here, we solved the cryogenic-electron microscopy (cryo-EM) structure of apo-ADGRG2, an ...Adhesion G protein-coupled receptors are elusive in terms of their structural information and ligands. Here, we solved the cryogenic-electron microscopy (cryo-EM) structure of apo-ADGRG2, an essential membrane receptor for maintaining male fertility, in complex with a G trimer. Whereas the formations of two kinks were determinants of the active state, identification of a potential ligand-binding pocket in ADGRG2 facilitated the screening and identification of dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate and deoxycorticosterone as potential ligands of ADGRG2. The cryo-EM structures of DHEA-ADGRG2-G provided interaction details for DHEA within the seven transmembrane domains of ADGRG2. Collectively, our data provide a structural basis for the activation and signaling of ADGRG2, as well as characterization of steroid hormones as ADGRG2 ligands, which might be used as useful tools for further functional studies of the orphan ADGRG2. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33249.map.gz emd_33249.map.gz | 46.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33249-v30.xml emd-33249-v30.xml emd-33249.xml emd-33249.xml | 25 KB 25 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33249.png emd_33249.png | 19.8 KB | ||
| Filedesc metadata |  emd-33249.cif.gz emd-33249.cif.gz | 7.7 KB | ||
| Others |  emd_33249_half_map_1.map.gz emd_33249_half_map_1.map.gz emd_33249_half_map_2.map.gz emd_33249_half_map_2.map.gz | 46.2 MB 46.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33249 http://ftp.pdbj.org/pub/emdb/structures/EMD-33249 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33249 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33249 | HTTPS FTP |
-Validation report
| Summary document |  emd_33249_validation.pdf.gz emd_33249_validation.pdf.gz | 866.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33249_full_validation.pdf.gz emd_33249_full_validation.pdf.gz | 866.4 KB | Display | |
| Data in XML |  emd_33249_validation.xml.gz emd_33249_validation.xml.gz | 12 KB | Display | |
| Data in CIF |  emd_33249_validation.cif.gz emd_33249_validation.cif.gz | 14.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33249 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33249 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33249 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33249 | HTTPS FTP |
-Related structure data
| Related structure data |  7xkeMC  7xkdC  7xkfC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33249.map.gz / Format: CCP4 / Size: 59.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33249.map.gz / Format: CCP4 / Size: 59.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The cryo-em density of DHEA-ADGRG2-FL-Gs complex | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.052 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: The half2 map of DHEA-ADGRG2-FL-Gs complex
| File | emd_33249_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The half2 map of DHEA-ADGRG2-FL-Gs complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: The half1 map of DHEA-ADGRG2-FL-Gs complex
| File | emd_33249_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The half1 map of DHEA-ADGRG2-FL-Gs complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Adhesion G-protein coupled receptor G2
| Entire | Name: Adhesion G-protein coupled receptor G2 |
|---|---|
| Components |
|
-Supramolecule #1: Adhesion G-protein coupled receptor G2
| Supramolecule | Name: Adhesion G-protein coupled receptor G2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 220 KDa |
-Macromolecule #1: mini-Gs
| Macromolecule | Name: mini-Gs / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.879465 KDa |
| Recombinant expression | Organism:  Baculovirus expression vector pFastBac1-HM Baculovirus expression vector pFastBac1-HM |
| Sequence | String: MGCTLSAEDK AAVERSKMIE KQLQKDKQVY RATHRLLLLG ADNSGKSTIV KQMRIYHVNG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMIE KQLQKDKQVY RATHRLLLLG ADNSGKSTIV KQMRIYHVNG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTSGIFETK FQVDKVNFHM FDVGAQRDER RKWIQCFNDV TAIIFVVDSS DYNRLQEALN DF KSIWNNR WLRTISVILF LNKQDLLAEK VLAGKSKIED YFPEFARYTT PEDATPEPGE DPRVTRAKYF IRDEFLRIST ASG DGRHYC YPHFTCSVDT ENARRIFNDC RDIIQRMHLR QYELL |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.48916 KDa |
| Recombinant expression | Organism:  Baculovirus expression vector pFastBac1-HM Baculovirus expression vector pFastBac1-HM |
| Sequence | String: MHHHHHHLEV LFQGPGSSQS ELDQLRQEAE QLKNQIRDAR KACADATLSQ ITNNIDPVGR IQMRTRRTLR GHLAKIYAMH WGTDSRLLV SASQDGKLII WDSYTTNKVH AIPLRSSWVM TCAYAPSGNY VACGGLDNIC SIYNLKTREG NVRVSRELAG H TGYLSCCR ...String: MHHHHHHLEV LFQGPGSSQS ELDQLRQEAE QLKNQIRDAR KACADATLSQ ITNNIDPVGR IQMRTRRTLR GHLAKIYAMH WGTDSRLLV SASQDGKLII WDSYTTNKVH AIPLRSSWVM TCAYAPSGNY VACGGLDNIC SIYNLKTREG NVRVSRELAG H TGYLSCCR FLDDNQIVTS SGDTTCALWD IETGQQTTTF TGHTGDVMSL SLAPDTRLFV SGACDASAKL WDVREGMCRQ TF TGHESDI NAICFFPNGN AFATGSDDAT CRLFDLRADQ ELMTYSHDNI ICGITSVSFS KSGRLLLAGY DDFNCNVWDA LKA DRAGVL AGHDNRVSCL GVTDDGMAVA TGSWDSFLKI WN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 6.375332 KDa |
| Recombinant expression | Organism:  Baculovirus expression vector pFastBac1-HM Baculovirus expression vector pFastBac1-HM |
| Sequence | String: NTASIAQARK LVEQLKMEAN IDRIKVSKAA ADLMAYCEAH AKEDPLLTPV PASENPFR UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: NB35
| Macromolecule | Name: NB35 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 13.885439 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGGG LVQPGGSLRL SCAASGFTFS NYKMNWVRQA PGKGLEWVSD ISQSGASISY TGSVKGRFTI SRDNAKNTLY LQMNSLKPE DTAVYYCARC PAPFTRDCFD VTSTTYAYRG QGTQVTVSS |
-Macromolecule #5: Adhesion G-protein coupled receptor G2
| Macromolecule | Name: Adhesion G-protein coupled receptor G2 / type: protein_or_peptide / ID: 5 Details: C-terminal(892-916) is due to the replacement of GPR120 tail. Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 98.934797 KDa |
| Recombinant expression | Organism:  Baculovirus expression vector pFastBac1-HM Baculovirus expression vector pFastBac1-HM |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDKLKENGN SSLLSPSAES SLVSLIPYSN GTPDAASEVL STLNKTEKSK ITIVKTFNAS GVKSQRNIC NLSSLCNDSV FFRGEIVFQH DEDHNVTQNQ DTANGTFAGV LSLSELKRSE LNKTLQTLSE TYFIVCATAE A QSTVNCTF ...String: MKTIIALSYI FCLVFADYKD DDDKLKENGN SSLLSPSAES SLVSLIPYSN GTPDAASEVL STLNKTEKSK ITIVKTFNAS GVKSQRNIC NLSSLCNDSV FFRGEIVFQH DEDHNVTQNQ DTANGTFAGV LSLSELKRSE LNKTLQTLSE TYFIVCATAE A QSTVNCTF TVKLNETMNV CAMMVTFQTV QIRPMEQCCC SPRTPCPSSP EELEKLQCEL QDPIVCLADQ PHGPPLSSSS KP VVPQATI ISHVASDFSL AEPLDHALMT PSTPSLTQES NLPSPQPTIP LASSPATDLP VQSVVVSSLP QTDLSHTLSP VQS SIPSPT TPAPSVPTEL VTISTPPGET VVNTSTVSDL EAQVSQMEKA LSLGSLEPNL AGEMVNRVSK LLHSPPALLA PLAQ RLLKV VDAIGLQLNF SSTTISLTSP SLALAVIRVN ASNFNTTTFA AQDPTNLQVS LETPPPENSI GAITLPSSLM NNLPA NDVE LASRIQFNFF ETPALFQDPS LENLTLISYV ISSSVTNMTI KNLTRNVTVA LKHINPSPDD LTVKCVFWDL GRNGGK GGW SSDGCSVKDK RMNETICTCS ALASFGILLD LSRTSLPPSQ MMALTFITYI GCGLSSIFLS VTLVTYIAFE KIRRDYP SK ILIQLCAALL LLNLIFLLDS WIALYNTRGF CIAVAVFLHY FLLVSFTWMG LEAFHMYLAL VKVFNTYIRK YILKFCIV G WGIPAVVVSI VLTISPDNYG IGSYGKFPNG TPDDFCWINS NVVFYITVVG YFCVIFLLNV SMFIVVLVQL CRIKKKKQL GAQRKTSIQD LRSIAGLTFL LGITWGFAFF AWGPVNVTFM YLFAIFNTLQ GFFIFIFYCA AKENVRKQWR RYLCCGKLFW FPEKGAILT DTSVKRNDLS IISG UniProtKB: Adhesion G-protein coupled receptor G2 |
-Macromolecule #6: 3-BETA-HYDROXY-5-ANDROSTEN-17-ONE
| Macromolecule | Name: 3-BETA-HYDROXY-5-ANDROSTEN-17-ONE / type: ligand / ID: 6 / Number of copies: 1 / Formula: AND |
|---|---|
| Molecular weight | Theoretical: 288.424 Da |
| Chemical component information | 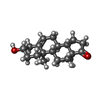 ChemComp-AND: |
-Macromolecule #7: water
| Macromolecule | Name: water / type: ligand / ID: 7 / Number of copies: 1 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 IS (4k x 4k) / Average electron dose: 58.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)