[English] 日本語
 Yorodumi
Yorodumi- EMDB-3319: CryoEM structure of the CMG replicative helicase bound to a DNA f... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3319 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of the CMG replicative helicase bound to a DNA fork (relaxed state) | |||||||||
 Map data Map data | CMG treated with forked DNA substrate in the presence of ATPgS in a relaxed Mcm5-2 AAA+ configuration | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Cdc45 / GINS / MCM / CMG / helicase / DNA replication | |||||||||
| Function / homology |  Function and homology information Function and homology informationUnwinding of DNA / Assembly of the pre-replicative complex / Switching of origins to a post-replicative state / DNA endoreduplication / Activation of ATR in response to replication stress / Activation of the pre-replicative complex / eggshell chorion gene amplification / Orc1 removal from chromatin / DNA amplification / DNA strand elongation involved in mitotic DNA replication ...Unwinding of DNA / Assembly of the pre-replicative complex / Switching of origins to a post-replicative state / DNA endoreduplication / Activation of ATR in response to replication stress / Activation of the pre-replicative complex / eggshell chorion gene amplification / Orc1 removal from chromatin / DNA amplification / DNA strand elongation involved in mitotic DNA replication / GINS complex / mitotic DNA replication preinitiation complex assembly / resolution of meiotic recombination intermediates / premeiotic DNA replication / mitotic DNA replication / CMG complex / DNA replication preinitiation complex / MCM complex / double-strand break repair via break-induced replication / mitotic DNA replication initiation / chromosome condensation / DNA strand elongation involved in DNA replication / DNA replication origin binding / DNA replication initiation / DNA helicase activity / regulation of DNA-templated transcription elongation / mitotic spindle organization / meiotic cell cycle / helicase activity / mitotic cell cycle / single-stranded DNA binding / DNA helicase / DNA replication / cell division / chromatin binding / ATP hydrolysis activity / zinc ion binding / ATP binding / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
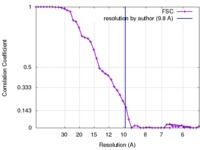
| Method | single particle reconstruction / cryo EM / Resolution: 9.8 Å | |||||||||
 Authors Authors | Abid Ali F / Renault L / Costa A | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2016 Journal: Nat Commun / Year: 2016Title: Cryo-EM structures of the eukaryotic replicative helicase bound to a translocation substrate. Authors: Ferdos Abid Ali / Ludovic Renault / Julian Gannon / Hailey L Gahlon / Abhay Kotecha / Jin Chuan Zhou / David Rueda / Alessandro Costa /  Abstract: The Cdc45-MCM-GINS (CMG) helicase unwinds DNA during the elongation step of eukaryotic genome duplication and this process depends on the MCM ATPase function. Whether CMG translocation occurs on ...The Cdc45-MCM-GINS (CMG) helicase unwinds DNA during the elongation step of eukaryotic genome duplication and this process depends on the MCM ATPase function. Whether CMG translocation occurs on single- or double-stranded DNA and how ATP hydrolysis drives DNA unwinding remain open questions. Here we use cryo-electron microscopy to describe two subnanometre resolution structures of the CMG helicase trapped on a DNA fork. In the predominant state, the ring-shaped C-terminal ATPase of MCM is compact and contacts single-stranded DNA, via a set of pre-sensor 1 hairpins that spiral around the translocation substrate. In the second state, the ATPase module is relaxed and apparently substrate free, while DNA intimately contacts the downstream amino-terminal tier of the MCM motor ring. These results, supported by single-molecule FRET measurements, lead us to suggest a replication fork unwinding mechanism whereby the N-terminal and AAA+ tiers of the MCM work in concert to translocate on single-stranded DNA. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
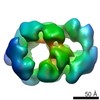
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3319.map.gz emd_3319.map.gz | 599.7 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3319-v30.xml emd-3319-v30.xml emd-3319.xml emd-3319.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3319_fsc.xml emd_3319_fsc.xml | 4.1 KB | Display |  FSC data file FSC data file |
| Images |  EMD-3319.png EMD-3319.png | 1.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3319 http://ftp.pdbj.org/pub/emdb/structures/EMD-3319 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3319 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3319 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3319.map.gz / Format: CCP4 / Size: 5.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3319.map.gz / Format: CCP4 / Size: 5.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CMG treated with forked DNA substrate in the presence of ATPgS in a relaxed Mcm5-2 AAA+ configuration | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.7 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : CMG treated with forked DNA substrate and ATPgS
+Supramolecule #1000: CMG treated with forked DNA substrate and ATPgS
+Macromolecule #1: Mcm2
+Macromolecule #4: Mcm3
+Macromolecule #5: Mcm4
+Macromolecule #6: Mcm5
+Macromolecule #7: Mcm6
+Macromolecule #8: Mcm7
+Macromolecule #9: Cdc45
+Macromolecule #10: Psf1
+Macromolecule #11: Psf2
+Macromolecule #12: Psf3
+Macromolecule #13: Sld5
+Macromolecule #2: Model replication DNA fork leading strand template
+Macromolecule #3: Model replication DNA fork lagging strand template
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 Details: 25 mM Hepes, 50 mM sodium acetate, 10 mM magnesium acetate, 1 mM DTT, 0.1mM ATPgammaS |
|---|---|
| Grid | Details: Quantifoil 1.2/1.3 or C-flat 1/1 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV / Method: Blot for 4 seconds before plunging |
- Electron microscopy #1
Electron microscopy #1
| Microscopy ID | 1 |
|---|---|
| Microscope | FEI POLARA 300 |
| Specialist optics | Energy filter - Name: GIF / Energy filter - Lower energy threshold: 0.0 eV / Energy filter - Upper energy threshold: 20.0 eV |
| Date | Aug 7, 2015 |
| Image recording | Category: CCD / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Digitization - Sampling interval: 5 µm / Number real images: 2098 / Average electron dose: 48 e/Å2 / Details: data was recorded as movies of 25 frames / Bits/pixel: 32 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.7 µm / Nominal magnification: 37037 |
| Sample stage | Specimen holder: Multi cartridge holder / Specimen holder model: OTHER |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Electron microscopy #2
Electron microscopy #2
| Microscopy ID | 2 |
|---|---|
| Microscope | FEI POLARA 300 |
| Specialist optics | Energy filter - Name: GIF / Energy filter - Lower energy threshold: 0.0 eV / Energy filter - Upper energy threshold: 20.0 eV |
| Date | Aug 7, 2015 |
| Image recording | Category: CCD / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Digitization - Sampling interval: 5 µm / Number real images: 2098 / Average electron dose: 48 e/Å2 / Details: data was recorded as movies of 25 frames / Bits/pixel: 32 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.7 µm / Nominal magnification: 37037 |
| Sample stage | Specimen holder: Multi cartridge holder / Specimen holder model: OTHER |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















 unidentified baculovirus
unidentified baculovirus