[English] 日本語
 Yorodumi
Yorodumi- EMDB-32757: Cryo-EM structure of SARS-CoV spike receptor-binding domain in co... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of SARS-CoV spike receptor-binding domain in complex with sea lion ACE2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Complex / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationMaturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / Hydrolases; Acting on peptide bonds (peptidases) / peptidyl-dipeptidase activity / carboxypeptidase activity / Attachment and Entry / metallopeptidase activity / SARS-CoV-1 activates/modulates innate immune responses / symbiont-mediated-mediated suppression of host tetherin activity ...Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / Hydrolases; Acting on peptide bonds (peptidases) / peptidyl-dipeptidase activity / carboxypeptidase activity / Attachment and Entry / metallopeptidase activity / SARS-CoV-1 activates/modulates innate immune responses / symbiont-mediated-mediated suppression of host tetherin activity / membrane fusion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / cilium / symbiont-mediated suppression of host innate immune response / apical plasma membrane / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / host cell plasma membrane / virion membrane / proteolysis / extracellular space / metal ion binding / identical protein binding / membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species | Mammalia (mammals) /  Severe acute respiratory syndrome coronavirus Severe acute respiratory syndrome coronavirus | |||||||||
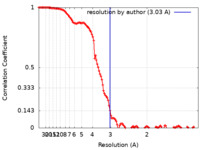
| Method | single particle reconstruction / cryo EM / Resolution: 3.03 Å | |||||||||
 Authors Authors | Li S / Han P | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Natl Sci Rev / Year: 2022 Journal: Natl Sci Rev / Year: 2022Title: Cross-species recognition and molecular basis of SARS-CoV-2 and SARS-CoV binding to ACE2s of marine animals. Authors: Shihua Li / Ruirui Yang / Di Zhang / Pu Han / Zepeng Xu / Qian Chen / Runchu Zhao / Xin Zhao / Xiao Qu / Anqi Zheng / Liang Wang / Linjie Li / Yu Hu / Rong Zhang / Chao Su / Sheng Niu / ...Authors: Shihua Li / Ruirui Yang / Di Zhang / Pu Han / Zepeng Xu / Qian Chen / Runchu Zhao / Xin Zhao / Xiao Qu / Anqi Zheng / Liang Wang / Linjie Li / Yu Hu / Rong Zhang / Chao Su / Sheng Niu / Yanfang Zhang / Jianxun Qi / Kefang Liu / Qihui Wang / George F Gao /  Abstract: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has an extremely broad host range that includes hippopotami, which are phylogenetically closely related to whales. The cellular ACE2 ...Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has an extremely broad host range that includes hippopotami, which are phylogenetically closely related to whales. The cellular ACE2 receptor is one of the key determinants of the host range. Here, we found that ACE2s from several marine mammals and hippopotami could efficiently bind to the receptor-binding domain (RBD) of both SARS-CoV and SARS-CoV-2 and facilitate the transduction of SARS-CoV and SARS-CoV-2 pseudoviruses into ACE2-expressing cells. We further resolved the cryo-electron microscopy complex structures of the minke whale ACE2 and sea lion ACE2, respectively, bound to the RBDs, revealing that they have similar binding modes to human ACE2 when it comes to the SARS-CoV-2 RBD and SARS-CoV RBD. Our results indicate that marine mammals could potentially be new victims or virus carriers of SARS-CoV-2, which deserves further careful investigation and study. It will provide an early warning for the prospective monitoring of marine mammals. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32757.map.gz emd_32757.map.gz | 168 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32757-v30.xml emd-32757-v30.xml emd-32757.xml emd-32757.xml | 15.4 KB 15.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_32757_fsc.xml emd_32757_fsc.xml | 12.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_32757.png emd_32757.png | 99 KB | ||
| Filedesc metadata |  emd-32757.cif.gz emd-32757.cif.gz | 6.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32757 http://ftp.pdbj.org/pub/emdb/structures/EMD-32757 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32757 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32757 | HTTPS FTP |
-Validation report
| Summary document |  emd_32757_validation.pdf.gz emd_32757_validation.pdf.gz | 520.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32757_full_validation.pdf.gz emd_32757_full_validation.pdf.gz | 520.4 KB | Display | |
| Data in XML |  emd_32757_validation.xml.gz emd_32757_validation.xml.gz | 13.1 KB | Display | |
| Data in CIF |  emd_32757_validation.cif.gz emd_32757_validation.cif.gz | 17.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32757 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32757 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32757 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32757 | HTTPS FTP |
-Related structure data
| Related structure data |  7wsgMC  7wseC  7wsfC  7wshC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32757.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32757.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.669 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : SARS-CoV spike receptor-binding domain in complex with sea lion ACE2
| Entire | Name: SARS-CoV spike receptor-binding domain in complex with sea lion ACE2 |
|---|---|
| Components |
|
-Supramolecule #1: SARS-CoV spike receptor-binding domain in complex with sea lion ACE2
| Supramolecule | Name: SARS-CoV spike receptor-binding domain in complex with sea lion ACE2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism: Mammalia (mammals) |
-Supramolecule #2: SARS-CoV spike receptor-binding domain
| Supramolecule | Name: SARS-CoV spike receptor-binding domain / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Severe acute respiratory syndrome coronavirus Severe acute respiratory syndrome coronavirus |
-Supramolecule #3: sea lion ACE2
| Supramolecule | Name: sea lion ACE2 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|
-Macromolecule #1: Angiotensin-converting enzyme
| Macromolecule | Name: Angiotensin-converting enzyme / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: Hydrolases; Acting on peptide bonds (peptidases) |
|---|---|
| Source (natural) | Organism: Mammalia (mammals) |
| Molecular weight | Theoretical: 93.009812 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MLGSSWLLLS LAALTAARST TEDLVKTFLE KFNSEAEELS YQSSLASWNY NTNITDENVQ KMNDAGAKWS AFYEEQSKQA KTYPLEEIQ DSTVKRQLQA LQHSGSSVLS ADKSQRLNTI LNAMSTIYST GKACNPNNPQ ECLLLEPGLD DIMANSRDYN E RLWAWEGW ...String: MLGSSWLLLS LAALTAARST TEDLVKTFLE KFNSEAEELS YQSSLASWNY NTNITDENVQ KMNDAGAKWS AFYEEQSKQA KTYPLEEIQ DSTVKRQLQA LQHSGSSVLS ADKSQRLNTI LNAMSTIYST GKACNPNNPQ ECLLLEPGLD DIMANSRDYN E RLWAWEGW RSEVGKQLRP LYEEYVALKN EMARANNYED YGDYWRGDYE EEWTNGYNYS RDQLIKDVEQ TFTQIQPLYE HL HAYVRAK LMDTYPSHMS PTGCLPAHLL GDMWGRFWTN LYPLTVPFGQ KPNIDVTDTM VNQSWDARRI FEEAEKFFVS VGL PNMTQG FWENSMLTEP GDSRKVVCHP TAWDLGKHDF RIKMCTKVTM DDFLTAHHEM GHIQYDMAYA AQPFLLRNGA NEGF HEAVG EIMSLSAATP KHLKNIGLLP PGFSEDNETD INFLFKQALT IVGTLPFTYM LEKWRWMVFK GEIPKEQWMK KWWEM KRDL VGVVEPLPHD ETYCDPASLF HVANDYSFIR YYTRTIYQFQ FQEALCQIAK HEGPLHKCDI SNSSEAGQTL LQMLKL GRS KPWTLALYRV VGAKNMDVRP LLNYFDPLFT WLKEQNRNSF VGWNTDWSPY ADQSIKVRIS LKSALGEKAY EWNDNEM YL FRSSIAYAMR EYFSKVKNQM IPFVEDNVWV NNLKPRISFT FFVTSPGNMS DIIPRADVEE AIRMSRGRIN DAFRLDDN S LEFLGIQPTL EPPYQPPVTI WLIVFGVVMA VVVVGIVLLI FSGIRSRRKN DQATSEENPY ASVNLSKGEN NPGFQNVDD VQTSSF UniProtKB: Angiotensin-converting enzyme |
-Macromolecule #2: Spike protein S1
| Macromolecule | Name: Spike protein S1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Severe acute respiratory syndrome coronavirus Severe acute respiratory syndrome coronavirus |
| Molecular weight | Theoretical: 21.624326 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: NLCPFGEVFN ATKFPSVYAW ERKKISNCVA DYSVLYNSTF FSTFKCYGVS ATKLNDLCFS NVYADSFVVK GDDVRQIAPG QTGVIADYN YKLPDDFMGC VLAWNTRNID ATSTGNYNYK YRYLRHGKLR PFERDISNVP FSPDGKPCTP PALNCYWPLN D YGFYTTTG IGYQPYRVVV LSFEGSLEVL FQ UniProtKB: Spike glycoprotein |
-Macromolecule #3: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 3 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)