[English] 日本語
 Yorodumi
Yorodumi- EMDB-32755: Cryo-EM structure of SARS-CoV-2 spike receptor-binding domain com... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of SARS-CoV-2 spike receptor-binding domain complexed with its receptor minke whale ACE2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Complex / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationHydrolases; Acting on peptide bonds (peptidases) / peptidyl-dipeptidase activity / carboxypeptidase activity / metallopeptidase activity / symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space ...Hydrolases; Acting on peptide bonds (peptidases) / peptidyl-dipeptidase activity / carboxypeptidase activity / metallopeptidase activity / symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion / entry receptor-mediated virion attachment to host cell / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / cilium / symbiont-mediated suppression of host innate immune response / apical plasma membrane / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / proteolysis / extracellular space / metal ion binding / identical protein binding / membrane / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species | Mammalia (mammals) /  | |||||||||
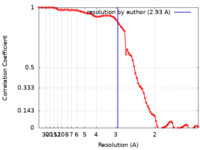
| Method | single particle reconstruction / cryo EM / Resolution: 2.93 Å | |||||||||
 Authors Authors | Li S / Han P | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Natl Sci Rev / Year: 2022 Journal: Natl Sci Rev / Year: 2022Title: Cross-species recognition and molecular basis of SARS-CoV-2 and SARS-CoV binding to ACE2s of marine animals. Authors: Shihua Li / Ruirui Yang / Di Zhang / Pu Han / Zepeng Xu / Qian Chen / Runchu Zhao / Xin Zhao / Xiao Qu / Anqi Zheng / Liang Wang / Linjie Li / Yu Hu / Rong Zhang / Chao Su / Sheng Niu / ...Authors: Shihua Li / Ruirui Yang / Di Zhang / Pu Han / Zepeng Xu / Qian Chen / Runchu Zhao / Xin Zhao / Xiao Qu / Anqi Zheng / Liang Wang / Linjie Li / Yu Hu / Rong Zhang / Chao Su / Sheng Niu / Yanfang Zhang / Jianxun Qi / Kefang Liu / Qihui Wang / George F Gao /  Abstract: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has an extremely broad host range that includes hippopotami, which are phylogenetically closely related to whales. The cellular ACE2 ...Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has an extremely broad host range that includes hippopotami, which are phylogenetically closely related to whales. The cellular ACE2 receptor is one of the key determinants of the host range. Here, we found that ACE2s from several marine mammals and hippopotami could efficiently bind to the receptor-binding domain (RBD) of both SARS-CoV and SARS-CoV-2 and facilitate the transduction of SARS-CoV and SARS-CoV-2 pseudoviruses into ACE2-expressing cells. We further resolved the cryo-electron microscopy complex structures of the minke whale ACE2 and sea lion ACE2, respectively, bound to the RBDs, revealing that they have similar binding modes to human ACE2 when it comes to the SARS-CoV-2 RBD and SARS-CoV RBD. Our results indicate that marine mammals could potentially be new victims or virus carriers of SARS-CoV-2, which deserves further careful investigation and study. It will provide an early warning for the prospective monitoring of marine mammals. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32755.map.gz emd_32755.map.gz | 117.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32755-v30.xml emd-32755-v30.xml emd-32755.xml emd-32755.xml | 14.6 KB 14.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_32755_fsc.xml emd_32755_fsc.xml | 8.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_32755.png emd_32755.png | 98.5 KB | ||
| Filedesc metadata |  emd-32755.cif.gz emd-32755.cif.gz | 6.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32755 http://ftp.pdbj.org/pub/emdb/structures/EMD-32755 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32755 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32755 | HTTPS FTP |
-Validation report
| Summary document |  emd_32755_validation.pdf.gz emd_32755_validation.pdf.gz | 511 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32755_full_validation.pdf.gz emd_32755_full_validation.pdf.gz | 510.6 KB | Display | |
| Data in XML |  emd_32755_validation.xml.gz emd_32755_validation.xml.gz | 11.1 KB | Display | |
| Data in CIF |  emd_32755_validation.cif.gz emd_32755_validation.cif.gz | 14.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32755 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32755 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32755 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32755 | HTTPS FTP |
-Related structure data
| Related structure data |  7wseMC  7wsfC  7wsgC  7wshC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32755.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32755.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.669 Å | ||||||||||||||||||||||||||||||||||||
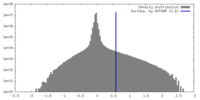
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : SARS-CoV-2 spike receptor-binding domain complexed with its recep...
| Entire | Name: SARS-CoV-2 spike receptor-binding domain complexed with its receptor minke whale ACE2 |
|---|---|
| Components |
|
-Supramolecule #1: SARS-CoV-2 spike receptor-binding domain complexed with its recep...
| Supramolecule | Name: SARS-CoV-2 spike receptor-binding domain complexed with its receptor minke whale ACE2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism: Mammalia (mammals) |
-Supramolecule #2: minke whale ACE2
| Supramolecule | Name: minke whale ACE2 / type: complex / ID: 2 / Parent: 1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: SARS-CoV-2 spike receptor-binding domain
| Supramolecule | Name: SARS-CoV-2 spike receptor-binding domain / type: complex / ID: 3 / Parent: 1 |
|---|
-Macromolecule #1: Angiotensin-converting enzyme
| Macromolecule | Name: Angiotensin-converting enzyme / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: Hydrolases; Acting on peptide bonds (peptidases) |
|---|---|
| Source (natural) | Organism: Mammalia (mammals) |
| Molecular weight | Theoretical: 85.789805 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGSFWLLLS LVAVTAAQST TEEQAKTFLQ KFDHEAEDLS YRSSLASWNY NTNITDENVQ KMNAARAKWS AFYEEQSRIA KTYPLEEIQ NLTLKRQLQA LQQSGTSVLS ADKSKRLNTI LNTMSTIYSS GKVLDPNTQE YLVLEPGLDD IMENSEDYNR R LWAWEGWR ...String: MSGSFWLLLS LVAVTAAQST TEEQAKTFLQ KFDHEAEDLS YRSSLASWNY NTNITDENVQ KMNAARAKWS AFYEEQSRIA KTYPLEEIQ NLTLKRQLQA LQQSGTSVLS ADKSKRLNTI LNTMSTIYSS GKVLDPNTQE YLVLEPGLDD IMENSEDYNR R LWAWEGWR AEVGKQLRPF YEEYVVLENE MARANNYEDY GDYWRGDYEV TGADGYDYSR NQLIADVERT FAEIKPLYEQ LH AYVRAKL MDAYPSRISP TGCLPAHLLG DMWGRFWTNL YPLTVPFGEK PSIDVTKEMQ NQSWDAKRIF KEAEKFFVSI GLP NMTQEF WVNSMLTEPG DGRKVVCHPT AWDLGKGDFR IKMCTKVTMD DFLTAHHEMG HIQYDMAYAT QPFLLRNGAN EGFH EAVGE IMSLSAATPH YLKALGLLPP DFYEDNVTEI NFLLKQALQI VGTLPFTYML EKWRWMVFKG EIPKEQWMQK WWEMK REIV GVVEPLPHDE TYCDPACLFH VAEDYSFIRY YTRTIYQFQF HEALCQTAKH EGPLYKCDIS NSTEAGQRLL QMLHLG KSE PWTLALENIV GVKTMDVKPL LNYFEPLLTW LKEQNRNSPV GWSTDWTPYS DQSIKVRISL KSALGEKAYE WNDNEMY LF QSSVAYAMRE YFSKVRNETI PFGEKDVWVS DLKPRISFNF FVTTPKNVSD IIPRTEVEEA IRMSRGRIND AFRLDDNS L EFLGIQPTLG PPYEPPVT UniProtKB: Angiotensin-converting enzyme |
-Macromolecule #2: Spike protein S1
| Macromolecule | Name: Spike protein S1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 21.873496 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: TNLCPFGEVF NATRFASVYA WNRKRISNCV ADYSVLYNSA SFSTFKCYGV SPTKLNDLCF TNVYADSFVI RGDEVRQIAP GQTGKIADY NYKLPDDFTG CVIAWNSNNL DSKVGGNYNY LYRLFRKSNL KPFERDISTE IYQAGSTPCN GVEGFNCYFP L QSYGFQPT ...String: TNLCPFGEVF NATRFASVYA WNRKRISNCV ADYSVLYNSA SFSTFKCYGV SPTKLNDLCF TNVYADSFVI RGDEVRQIAP GQTGKIADY NYKLPDDFTG CVIAWNSNNL DSKVGGNYNY LYRLFRKSNL KPFERDISTE IYQAGSTPCN GVEGFNCYFP L QSYGFQPT NGVGYQPYRV VVLSFELLHA PATVCGP UniProtKB: Spike glycoprotein |
-Macromolecule #3: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 3 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)