[English] 日本語
 Yorodumi
Yorodumi- EMDB-32211: Short chain dehydrogenase (SCR) cryoEM structure with NADP and et... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Short chain dehydrogenase (SCR) cryoEM structure with NADP and ethyl 4-chloroacetoacetate | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Rossman fold / tetramer / tag-free / wild type with NADPH / OXIDOREDUCTASE | |||||||||
| Function / homology | mannitol 2-dehydrogenase (NADP+) activity / oxidoreductase activity, acting on NAD(P)H, oxygen as acceptor / small molecule metabolic process / Oxidoreductases; Acting on the CH-OH group of donors; With NAD+ or NADP+ as acceptor / Enoyl-(Acyl carrier protein) reductase / Short-chain dehydrogenase/reductase SDR / carbohydrate metabolic process / NAD(P)-binding domain superfamily / Carbonyl Reductase Function and homology information Function and homology information | |||||||||
| Biological species |  Candida parapsilosis (yeast) Candida parapsilosis (yeast) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.13 Å | |||||||||
 Authors Authors | Li YH / Zhang RZ | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2022 Journal: EMBO J / Year: 2022Title: Oligomeric interactions maintain active-site structure in a noncooperative enzyme family. Authors: Yaohui Li / Rongzhen Zhang / Chi Wang / Farhad Forouhar / Oliver B Clarke / Sergey Vorobiev / Shikha Singh / Gaetano T Montelione / Thomas Szyperski / Yan Xu / John F Hunt /   Abstract: The evolutionary benefit accounting for widespread conservation of oligomeric structures in proteins lacking evidence of intersubunit cooperativity remains unclear. Here, crystal and cryo-EM ...The evolutionary benefit accounting for widespread conservation of oligomeric structures in proteins lacking evidence of intersubunit cooperativity remains unclear. Here, crystal and cryo-EM structures, and enzymological data, demonstrate that a conserved tetramer interface maintains the active-site structure in one such class of proteins, the short-chain dehydrogenase/reductase (SDR) superfamily. Phylogenetic comparisons support a significantly longer polypeptide being required to maintain an equivalent active-site structure in the context of a single subunit. Oligomerization therefore enhances evolutionary fitness by reducing the metabolic cost of enzyme biosynthesis. The large surface area of the structure-stabilizing oligomeric interface yields a synergistic gain in fitness by increasing tolerance to activity-enhancing yet destabilizing mutations. We demonstrate that two paralogous SDR superfamily enzymes with different specificities can form mixed heterotetramers that combine their individual enzymological properties. This suggests that oligomerization can also diversify the functions generated by a given metabolic investment, enhancing the fitness advantage provided by this architectural strategy. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32211.map.gz emd_32211.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32211-v30.xml emd-32211-v30.xml emd-32211.xml emd-32211.xml | 12 KB 12 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32211.png emd_32211.png | 208.2 KB | ||
| Filedesc metadata |  emd-32211.cif.gz emd-32211.cif.gz | 5.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32211 http://ftp.pdbj.org/pub/emdb/structures/EMD-32211 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32211 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32211 | HTTPS FTP |
-Related structure data
| Related structure data |  7vyqMC  7dldC  7dllC  7dlmC  7dmgC  7dn1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
| EM raw data |  EMPIAR-10872 (Title: Oligomeric interactions maintain active-site structure in a non-cooperative enzyme family EMPIAR-10872 (Title: Oligomeric interactions maintain active-site structure in a non-cooperative enzyme familyData size: 902.0 Data #1: Native-SCR-tetramer-frames [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32211.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32211.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.837 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Ternary complex of SCR with NADP and ethyl 4-chloroacetoacetate
| Entire | Name: Ternary complex of SCR with NADP and ethyl 4-chloroacetoacetate |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of SCR with NADP and ethyl 4-chloroacetoacetate
| Supramolecule | Name: Ternary complex of SCR with NADP and ethyl 4-chloroacetoacetate type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Candida parapsilosis (yeast) Candida parapsilosis (yeast) |
-Macromolecule #1: Carbonyl Reductase
| Macromolecule | Name: Carbonyl Reductase / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO EC number: Oxidoreductases; Acting on the CH-OH group of donors; With NAD+ or NADP+ as acceptor |
|---|---|
| Source (natural) | Organism:  Candida parapsilosis (yeast) Candida parapsilosis (yeast) |
| Molecular weight | Theoretical: 30.204873 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SMGEIESYCN KELGPLPTKA PTLSKNVLDL FSLKGKVASV TGSSGGIGWA VAEAYAQAGA DVAIWYNSHP ADEKAEHLQK TYGVHSKAY KCNISDPKSV EETISQQEKD FGTIDVFVAN AGVTWTQGPE IDVDNYDSWN KIISVDLNGV YYCSHNIGKI F KKNGKGSL ...String: SMGEIESYCN KELGPLPTKA PTLSKNVLDL FSLKGKVASV TGSSGGIGWA VAEAYAQAGA DVAIWYNSHP ADEKAEHLQK TYGVHSKAY KCNISDPKSV EETISQQEKD FGTIDVFVAN AGVTWTQGPE IDVDNYDSWN KIISVDLNGV YYCSHNIGKI F KKNGKGSL IITSSISGKI VNIPQLQAPY NTAKAACTHL AKSLAIEWAP FARVNTISPG YIDTDITDFA SKDMKAKWWQ LT PLGREGL TQELVGGYLY LASNASTFTT GSDVVIDGGY TCP UniProtKB: Carbonyl Reductase |
-Macromolecule #2: NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE
| Macromolecule | Name: NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE / type: ligand / ID: 2 / Number of copies: 4 / Formula: NAP |
|---|---|
| Molecular weight | Theoretical: 743.405 Da |
| Chemical component information |  ChemComp-NAP: |
-Macromolecule #3: ethyl 4-chloranyl-3-oxidanylidene-butanoate
| Macromolecule | Name: ethyl 4-chloranyl-3-oxidanylidene-butanoate / type: ligand / ID: 3 / Number of copies: 4 / Formula: 83I |
|---|---|
| Molecular weight | Theoretical: 164.587 Da |
| Chemical component information | 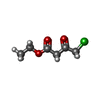 ChemComp-83I: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL |
|---|---|
| Buffer | pH: 6 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 2885 / Average exposure time: 2.5 sec. / Average electron dose: 56.15 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.17 µm / Nominal defocus min: 1.37 µm / Nominal magnification: 105000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY |
|---|---|
| Final reconstruction | Applied symmetry - Point group: D2 (2x2 fold dihedral) / Resolution.type: BY AUTHOR / Resolution: 3.13 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 104278 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















