[English] 日本語
 Yorodumi
Yorodumi- EMDB-3143: Electron microscopy of flagellin/NAIP5/CARD-truncated NLRC4(R288A) -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3143 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Electron microscopy of flagellin/NAIP5/CARD-truncated NLRC4(R288A) | |||||||||
 Map data Map data | Reconstruction of flagellin/NAIP5/CARD-truncated NLRC4(R288A) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | inflammasome / NLRC4 / NAIP | |||||||||
| Biological species |   Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) | |||||||||
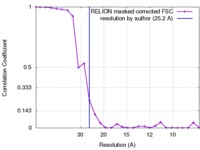
| Method | single particle reconstruction / negative staining / Resolution: 25.2 Å | |||||||||
 Authors Authors | Hu ZH / Zhou Q / Zhang CL / Fan SL / Cheng W / Zhao Y / Shao F / Wang HW / Sui SF / Chai JJ | |||||||||
 Citation Citation |  Journal: Science / Year: 2015 Journal: Science / Year: 2015Title: Structural and biochemical basis for induced self-propagation of NLRC4. Authors: Zehan Hu / Qiang Zhou / Chenlu Zhang / Shilong Fan / Wei Cheng / Yue Zhao / Feng Shao / Hong-Wei Wang / Sen-Fang Sui / Jijie Chai /  Abstract: Responding to stimuli, nucleotide-binding domain and leucine-rich repeat-containing proteins (NLRs) oligomerize into multiprotein complexes, termed inflammasomes, mediating innate immunity. ...Responding to stimuli, nucleotide-binding domain and leucine-rich repeat-containing proteins (NLRs) oligomerize into multiprotein complexes, termed inflammasomes, mediating innate immunity. Recognition of bacterial pathogens by NLR apoptosis inhibitory proteins (NAIPs) induces NLR family CARD domain-containing protein 4 (NLRC4) activation and formation of NAIP-NLRC4 inflammasomes. The wheel-like structure of a PrgJ-NAIP2-NLRC4 complex determined by cryogenic electron microscopy at 6.6 angstrom reveals that NLRC4 activation involves substantial structural reorganization that creates one oligomerization surface (catalytic surface). Once activated, NLRC4 uses this surface to catalyze the activation of an inactive NLRC4, self-propagating its active conformation to form the wheel-like architecture. NAIP proteins possess a catalytic surface matching the other oligomerization surface (receptor surface) of NLRC4 but not those of their own, ensuring that one NAIP is sufficient to initiate NLRC4 oligomerization. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3143.map.gz emd_3143.map.gz | 788.3 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3143-v30.xml emd-3143-v30.xml emd-3143.xml emd-3143.xml | 10.9 KB 10.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3143_fsc.xml emd_3143_fsc.xml | 2.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_3143.png emd_3143.png | 114.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3143 http://ftp.pdbj.org/pub/emdb/structures/EMD-3143 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3143 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3143 | HTTPS FTP |
-Validation report
| Summary document |  emd_3143_validation.pdf.gz emd_3143_validation.pdf.gz | 242.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_3143_full_validation.pdf.gz emd_3143_full_validation.pdf.gz | 241.2 KB | Display | |
| Data in XML |  emd_3143_validation.xml.gz emd_3143_validation.xml.gz | 6.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3143 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3143 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3143 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3143 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_3143.map.gz / Format: CCP4 / Size: 825.2 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3143.map.gz / Format: CCP4 / Size: 825.2 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of flagellin/NAIP5/CARD-truncated NLRC4(R288A) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Flagellin/NAIP5/CARD-truncated NLRC4(R288A) inflammasome
| Entire | Name: Flagellin/NAIP5/CARD-truncated NLRC4(R288A) inflammasome |
|---|---|
| Components |
|
-Supramolecule #1000: Flagellin/NAIP5/CARD-truncated NLRC4(R288A) inflammasome
| Supramolecule | Name: Flagellin/NAIP5/CARD-truncated NLRC4(R288A) inflammasome type: sample / ID: 1000 / Oligomeric state: Flagellin:NAIP5:NLRC4 = 1:1:1 / Number unique components: 3 |
|---|---|
| Molecular weight | Experimental: 250 KDa / Theoretical: 250 KDa |
-Macromolecule #1: NLRC4
| Macromolecule | Name: NLRC4 / type: protein_or_peptide / ID: 1 / Name.synonym: IPAF / Number of copies: 1 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 100 KDa / Theoretical: 100 KDa |
| Recombinant expression | Organism:  |
-Macromolecule #2: Flagellin
| Macromolecule | Name: Flagellin / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) |
| Recombinant expression | Organism:  |
-Macromolecule #3: NAIP5
| Macromolecule | Name: NAIP5 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.05 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 25 mM Tris-HCl pH 8.0 and 150 mM NaCl |
| Staining | Type: NEGATIVE Details: Grids with adsorbed protein staind with 2% w/v uranyl acetate for 60 seconds. |
| Grid | Details: 200 mesh copper grid with thin carbon film |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Date | Dec 22, 2014 |
| Image recording | Category: CCD / Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Number real images: 106 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 133588 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 80000 |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: |
|---|---|
| Software | Name:  Chimera Chimera |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)