[English] 日本語
 Yorodumi
Yorodumi- EMDB-30795: Structure of COVID-19 RNA-dependent RNA polymerase (extended conf... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30795 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
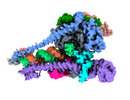
| Title | Structure of COVID-19 RNA-dependent RNA polymerase (extended conformation) bound to penciclovir | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | COVID-19 / RNA polymerase in extended conformation / penciclovir binding / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationprotein guanylyltransferase activity / RNA endonuclease activity producing 3'-phosphomonoesters, hydrolytic mechanism / mRNA guanylyltransferase activity / 5'-3' RNA helicase activity / Lyases; Phosphorus-oxygen lyases / Assembly of the SARS-CoV-2 Replication-Transcription Complex (RTC) / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of TBK1 activity / Maturation of replicase proteins / TRAF3-dependent IRF activation pathway / ISG15-specific peptidase activity ...protein guanylyltransferase activity / RNA endonuclease activity producing 3'-phosphomonoesters, hydrolytic mechanism / mRNA guanylyltransferase activity / 5'-3' RNA helicase activity / Lyases; Phosphorus-oxygen lyases / Assembly of the SARS-CoV-2 Replication-Transcription Complex (RTC) / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of TBK1 activity / Maturation of replicase proteins / TRAF3-dependent IRF activation pathway / ISG15-specific peptidase activity / Transcription of SARS-CoV-2 sgRNAs / Translation of Replicase and Assembly of the Replication Transcription Complex / snRNP Assembly / Replication of the SARS-CoV-2 genome / Hydrolases; Acting on ester bonds; Exoribonucleases producing 5'-phosphomonoesters / host cell endoplasmic reticulum-Golgi intermediate compartment / double membrane vesicle viral factory outer membrane / SARS coronavirus main proteinase / 5'-3' DNA helicase activity / 3'-5'-RNA exonuclease activity / host cell endosome / symbiont-mediated degradation of host mRNA / mRNA guanylyltransferase / symbiont-mediated suppression of host ISG15-protein conjugation / G-quadruplex RNA binding / symbiont-mediated suppression of host toll-like receptor signaling pathway / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of IRF3 activity / omega peptidase activity / mRNA (guanine-N7)-methyltransferase / SARS-CoV-2 modulates host translation machinery / methyltransferase cap1 / host cell Golgi apparatus / symbiont-mediated suppression of host NF-kappaB cascade / symbiont-mediated perturbation of host ubiquitin-like protein modification / DNA helicase / methyltransferase cap1 activity / ubiquitinyl hydrolase 1 / cysteine-type deubiquitinase activity / mRNA 5'-cap (guanine-N7-)-methyltransferase activity / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / single-stranded RNA binding / host cell perinuclear region of cytoplasm / regulation of autophagy / viral protein processing / lyase activity / host cell endoplasmic reticulum membrane / RNA helicase / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / symbiont-mediated suppression of host gene expression / copper ion binding / viral translational frameshifting / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / lipid binding / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell nucleus / ATP hydrolysis activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.73 Å | |||||||||
 Authors Authors | Li Z / Yu X | |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Structural basis for repurpose and design of nucleotide drugs for treating COVID-19 Authors: Yin W / Luan X / Li Z / Xie Y / Zhou Z / Liu J / Gao M / Wang X / Zhou F / Wang Q / Wang Q / Shen D / Zhang Y / Tian G / Aisa H / Wei D / Jiang Y / Xiao G / Jiang H / Zhang L / Yu X / Shen J / Zhang S / Xu H | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30795.map.gz emd_30795.map.gz | 60 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30795-v30.xml emd-30795-v30.xml emd-30795.xml emd-30795.xml | 18.3 KB 18.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30795.png emd_30795.png | 146.1 KB | ||
| Filedesc metadata |  emd-30795.cif.gz emd-30795.cif.gz | 6.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30795 http://ftp.pdbj.org/pub/emdb/structures/EMD-30795 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30795 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30795 | HTTPS FTP |
-Validation report
| Summary document |  emd_30795_validation.pdf.gz emd_30795_validation.pdf.gz | 622.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_30795_full_validation.pdf.gz emd_30795_full_validation.pdf.gz | 622.4 KB | Display | |
| Data in XML |  emd_30795_validation.xml.gz emd_30795_validation.xml.gz | 6 KB | Display | |
| Data in CIF |  emd_30795_validation.cif.gz emd_30795_validation.cif.gz | 6.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30795 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30795 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30795 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30795 | HTTPS FTP |
-Related structure data
| Related structure data |  7dokMC  7dfgC  7dfhC  7doiC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30795.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30795.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.069 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : COVID-19 RdRp complex (exntended conformation) bound to penciclovir
+Supramolecule #1: COVID-19 RdRp complex (exntended conformation) bound to penciclovir
+Macromolecule #1: RNA (5'-R(P*GP*CP*UP*AP*UP*GP*UP*GP*AP*GP*AP*UP*UP*AP*AP*GP*UP*UP...
+Macromolecule #2: RNA (5'-R(P*CP*CP*CP*UP*AP*UP*AP*AP*CP*UP*UP*AP*AP*UP*CP*UP*CP*AP...
+Macromolecule #3: RNA-directed RNA polymerase
+Macromolecule #4: Non-structural protein 8
+Macromolecule #5: Non-structural protein 7
+Macromolecule #6: [(2R)-4-(2-azanyl-6-oxidanylidene-3H-purin-9-yl)-2-(hydroxymethyl...
+Macromolecule #7: MAGNESIUM ION
+Macromolecule #8: ZINC ION
+Macromolecule #9: PYROPHOSPHATE 2-
+Macromolecule #10: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 68.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)