+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30583 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of cotton cellulose synthase isoform 7 | |||||||||
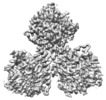
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | cellulose synthase / cotton / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationcellulose synthase activity / plant-type primary cell wall biogenesis / cellulose synthase (UDP-forming) / cellulose synthase (UDP-forming) activity / cellulose biosynthetic process / cell wall organization / zinc ion binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Guan ZY / Xue Y | |||||||||
 Citation Citation |  Journal: Plant Biotechnol J / Year: 2021 Journal: Plant Biotechnol J / Year: 2021Title: Structural insights into homotrimeric assembly of cellulose synthase CesA7 from Gossypium hirsutum. Authors: Xiangnan Zhang / Yuan Xue / Zeyuan Guan / Chen Zhou / Yangfan Nie / She Men / Qiang Wang / Cuicui Shen / Delin Zhang / Shuangxia Jin / Lili Tu / Ping Yin / Xianlong Zhang /  Abstract: Cellulose is one of the most abundant organic polymers in nature. It contains multiple β-1,4-glucan chains synthesized by cellulose synthases (CesAs) on the plasma membrane of higher plants. CesA ...Cellulose is one of the most abundant organic polymers in nature. It contains multiple β-1,4-glucan chains synthesized by cellulose synthases (CesAs) on the plasma membrane of higher plants. CesA subunits assemble into a pseudo-sixfold symmetric cellulose synthase complex (CSC), known as a 'rosette complex'. The structure of CesA remains enigmatic. Here, we report the cryo-EM structure of the homotrimeric CesA7 from Gossypium hirsutum at 3.5-angstrom resolution. The GhCesA7 homotrimer shows a C3 symmetrical assembly. Each protomer contains seven transmembrane helices (TMs) which form a channel potentially facilitating the release of newly synthesized glucans. The cytoplasmic glycosyltransferase domain (GT domain) of GhCesA7 protrudes from the membrane, and its catalytic pocket is directed towards the TM pore. The homotrimer GhCesA7 is stabilized by the transmembrane helix 7 (TM7) and the plant-conserved region (PCR) domains. It represents the building block of CSCs and facilitates microfibril formation. This structure provides insight into how eukaryotic cellulose synthase assembles and provides a mechanistic basis for the improvement of cotton fibre quality in the future. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30583.map.gz emd_30583.map.gz | 5.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30583-v30.xml emd-30583-v30.xml emd-30583.xml emd-30583.xml | 10.5 KB 10.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30583.png emd_30583.png | 87.4 KB | ||
| Filedesc metadata |  emd-30583.cif.gz emd-30583.cif.gz | 5.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30583 http://ftp.pdbj.org/pub/emdb/structures/EMD-30583 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30583 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30583 | HTTPS FTP |
-Validation report
| Summary document |  emd_30583_validation.pdf.gz emd_30583_validation.pdf.gz | 371.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_30583_full_validation.pdf.gz emd_30583_full_validation.pdf.gz | 370.7 KB | Display | |
| Data in XML |  emd_30583_validation.xml.gz emd_30583_validation.xml.gz | 6.4 KB | Display | |
| Data in CIF |  emd_30583_validation.cif.gz emd_30583_validation.cif.gz | 7.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30583 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30583 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30583 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30583 | HTTPS FTP |
-Related structure data
| Related structure data |  7d5kMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30583.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30583.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.087 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Cellulose synthase
| Entire | Name: Cellulose synthase |
|---|---|
| Components |
|
-Supramolecule #1: Cellulose synthase
| Supramolecule | Name: Cellulose synthase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Cellulose synthase
| Macromolecule | Name: Cellulose synthase / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO / EC number: cellulose synthase (UDP-forming) |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 118.129711 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MEASAGLVAG SHNRNELVVI HGHEEPKPLK NLDGQVCEIC GDEIGVTVDG DLFVACNECG FPVCRPCYEY ERREGTQQCP QCKTRYKRL KGSPRVEGDE DEEDVDDIEH EFNIDDEQNK HRNVVESILH GKMSYGRGPE DDETPQIPVI TGVRSRPVSG E FPIAGALA ...String: MEASAGLVAG SHNRNELVVI HGHEEPKPLK NLDGQVCEIC GDEIGVTVDG DLFVACNECG FPVCRPCYEY ERREGTQQCP QCKTRYKRL KGSPRVEGDE DEEDVDDIEH EFNIDDEQNK HRNVVESILH GKMSYGRGPE DDETPQIPVI TGVRSRPVSG E FPIAGALA YGEHMPNASL HKRVHPYPMS ETEGAERWDD KKEGGWKERM DDWKMQQGNL GPEADDAYDD MSMLDEARQP LS RKVPIAS SKINPYRMVI VARLLILAFF LRYRILNPVH DAIGLWLTSV ICEIWFAFSW ILDQFPKWFP IDRETYLDRL SLR YEREGE PNMLAPVDIF VSTVDPMKEP PLVTANTVLS ILAMDYPVDK ISCYISDDGA SMLTFESLSE TAEFARKWVP FCKK FAIEP RAPEMYFTLK VDYLKDKVQP TFVKERRAMK REYEEFKVRI NALVAKAQKV PPEGWIMQDG TPWPGNNTKD HPGMI QVFL GQSGGHDTEG NELPRLVYVS REKRPGFLHH KKAGAMNALV RVSGVLTNAP FMLNLDCDHY INNSKAAREA MCFLMD PQI GRKVCYVQFP QRFDGIDRHD RYANRNTVFF DINMKGLDGI QGPVYVGTGC VFRRQALYGY EPPKGPKRPK MVSCGCC PC FGRRKKDKKY PKNGGNENGP SLEAVEDDKE LLMSQMNFEK KFGQSAIFVT STLMDQGGVP PSSSPAALLK EAIHVISC G YEDKTEWGSE LGWIYGSITE DILTGFKMHC RGWRSIYCMP KLPAFKGSAP INLSDRLNQV LRWALGSVEI FFSRHCPAW YGLKGAKLRW LERFAYVNTT IYPFTSLPLL AYCTLPAICL LTDKFIMPPI STFASLFFIA LFLSIFATGI LELRWSGVSI EEWWRNEQF WVIGGISAHL FAVVQGLLKV LAGIDTNFTV TSKTTDDEEF GELYTFKWTT LLIPPTTVLI INLVGVVAGI S DAINNGYQ SWGPLFGKLF FSFWVIVHLY PFLKGLMGRQ NRTPTIVVIW SVLLASIFSL LWVRIDPFVL KTKGPDTTQC GI NC UniProtKB: Cellulose synthase |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















