+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30340 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the 2:2 cGAS-nucleosome complex | ||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
 Keywords Keywords | cGAS / nucleosome / inhibition / cryo-EM / IMMUNE SYSTEM / STRUCTURAL PROTEIN-TRANSFERASE-DNA complex | ||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology information2',3'-cyclic GMP-AMP synthase activity / cyclic GMP-AMP synthase / paracrine signaling / STING mediated induction of host immune responses / poly-ADP-D-ribose modification-dependent protein binding / regulation of immunoglobulin production / cGAS/STING signaling pathway / regulation of T cell activation / pattern recognition receptor signaling pathway / : ...2',3'-cyclic GMP-AMP synthase activity / cyclic GMP-AMP synthase / paracrine signaling / STING mediated induction of host immune responses / poly-ADP-D-ribose modification-dependent protein binding / regulation of immunoglobulin production / cGAS/STING signaling pathway / regulation of T cell activation / pattern recognition receptor signaling pathway / : / negative regulation of cGAS/STING signaling pathway / cytoplasmic pattern recognition receptor signaling pathway / cellular response to exogenous dsRNA / positive regulation of type I interferon production / negative regulation of tumor necrosis factor-mediated signaling pathway / : / negative regulation of double-strand break repair via homologous recombination / nucleosome binding / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / positive regulation of defense response to virus by host / phosphatidylinositol-4,5-bisphosphate binding / Packaging Of Telomere Ends / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / telomere organization / activation of innate immune response / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Interleukin-7 signaling / RNA Polymerase I Promoter Opening / epigenetic regulation of gene expression / Inhibition of DNA recombination at telomere / Assembly of the ORC complex at the origin of replication / Meiotic synapsis / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / determination of adult lifespan / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / innate immune response in mucosa / Defective pyroptosis / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / RNA Polymerase I Promoter Escape / Nonhomologous End-Joining (NHEJ) / molecular condensate scaffold activity / lipopolysaccharide binding / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / HDMs demethylate histones / G2/M DNA damage checkpoint / NoRC negatively regulates rRNA expression / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / DNA Damage/Telomere Stress Induced Senescence / Pre-NOTCH Transcription and Translation / Meiotic recombination / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Metalloprotease DUBs / RMTs methylate histone arginines / Transcriptional regulation of granulopoiesis / HCMV Early Events / antimicrobial humoral immune response mediated by antimicrobial peptide / structural constituent of chromatin / positive regulation of cellular senescence / UCH proteinases / antibacterial humoral response / heterochromatin formation / nucleosome / nucleosome assembly / E3 ubiquitin ligases ubiquitinate target proteins / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / site of double-strand break / HATs acetylate histones / RUNX1 regulates transcription of genes involved in differentiation of HSCs / Factors involved in megakaryocyte development and platelet production / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / chromatin organization / Processing of DNA double-strand break ends / Senescence-Associated Secretory Phenotype (SASP) / double-stranded DNA binding / Oxidative Stress Induced Senescence / defense response to Gram-negative bacterium / gene expression / defense response to virus / Estrogen-dependent gene expression / killing of cells of another organism Similarity search - Function | ||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.9 Å | ||||||||||||||||||||||||
 Authors Authors | Cao D / Han X | ||||||||||||||||||||||||
| Funding support |  China, 7 items China, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Cell Res / Year: 2020 Journal: Cell Res / Year: 2020Title: Structural basis for nucleosome-mediated inhibition of cGAS activity. Authors: Duanfang Cao / Xiaonan Han / Xiaoyi Fan / Rui-Ming Xu / Xinzheng Zhang /  Abstract: Activation of cyclic GMP-AMP synthase (cGAS) through sensing cytosolic double stranded DNA (dsDNA) plays a pivotal role in innate immunity against exogenous infection as well as cellular regulation ...Activation of cyclic GMP-AMP synthase (cGAS) through sensing cytosolic double stranded DNA (dsDNA) plays a pivotal role in innate immunity against exogenous infection as well as cellular regulation under stress. Aberrant activation of cGAS induced by self-DNA is related to autoimmune diseases. cGAS accumulates at chromosomes during mitosis or spontaneously in the nucleus. Binding of cGAS to the nucleosome competitively attenuates the dsDNA-mediated cGAS activation, but the molecular mechanism of the attenuation is still poorly understood. Here, we report two cryo-electron microscopy structures of cGAS-nucleosome complexes. The structures reveal that cGAS interacts with the nucleosome as a monomer, forming 1:1 and 2:2 complexes, respectively. cGAS contacts the nucleosomal acidic patch formed by the H2A-H2B heterodimer through the dsDNA-binding site B in both complexes, and could interact with the DNA from the other symmetrically placed nucleosome via the dsDNA-binding site C in the 2:2 complex. The bound nucleosome inhibits the activation of cGAS through blocking the interaction of cGAS with ligand dsDNA and disrupting cGAS dimerization. R236A or R255A mutation of cGAS impairs the binding between cGAS and the nucleosome, and largely relieves the nucleosome-mediated inhibition of cGAS activity. Our study provides structural insights into the inhibition of cGAS activity by the nucleosome, and advances the understanding of the mechanism by which hosts avoid the autoimmune attack caused by cGAS. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30340.map.gz emd_30340.map.gz | 85.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30340-v30.xml emd-30340-v30.xml emd-30340.xml emd-30340.xml | 22.9 KB 22.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30340.png emd_30340.png | 67.6 KB | ||
| Filedesc metadata |  emd-30340.cif.gz emd-30340.cif.gz | 6.8 KB | ||
| Others |  emd_30340_additional_1.map.gz emd_30340_additional_1.map.gz | 85.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30340 http://ftp.pdbj.org/pub/emdb/structures/EMD-30340 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30340 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30340 | HTTPS FTP |
-Validation report
| Summary document |  emd_30340_validation.pdf.gz emd_30340_validation.pdf.gz | 478.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_30340_full_validation.pdf.gz emd_30340_full_validation.pdf.gz | 478.4 KB | Display | |
| Data in XML |  emd_30340_validation.xml.gz emd_30340_validation.xml.gz | 5.7 KB | Display | |
| Data in CIF |  emd_30340_validation.cif.gz emd_30340_validation.cif.gz | 6.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30340 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30340 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30340 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30340 | HTTPS FTP |
-Related structure data
| Related structure data |  7ccrMC  7ccqC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30340.map.gz / Format: CCP4 / Size: 98.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30340.map.gz / Format: CCP4 / Size: 98.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
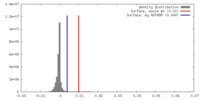
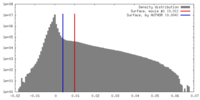
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: #1
| File | emd_30340_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
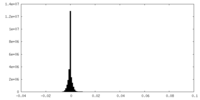
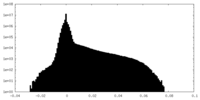
| Density Histograms |
- Sample components
Sample components
-Entire : the cGAS-nucleosome complex in 2:2 molar ratio
| Entire | Name: the cGAS-nucleosome complex in 2:2 molar ratio |
|---|---|
| Components |
|
-Supramolecule #1: the cGAS-nucleosome complex in 2:2 molar ratio
| Supramolecule | Name: the cGAS-nucleosome complex in 2:2 molar ratio / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#7 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Histone H3.1
| Macromolecule | Name: Histone H3.1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.504476 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: PHRYRPGTVA LREIRRYQKS TELLIRKLPF QRLVREIAQD FKTDLRFQSS AVMALQEACE AYLVGLFEDT NLCAIHAKRV TIMPKDIQL ARRIRGERA UniProtKB: Histone H3.1 |
-Macromolecule #2: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 9.067586 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: RDNIQGITKP AIRRLARRGG VKRISGLIYE ETRGVLKVFL ENVIRDAVTY TEHAKRKTVT AMDVVYALKR QGRTLYGFGG |
-Macromolecule #3: Histone H2A type 1-B/E
| Macromolecule | Name: Histone H2A type 1-B/E / type: protein_or_peptide / ID: 3 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.308161 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: KTRSSRAGLQ FPVGRVHRLL RKGNYSERVG AGAPVYLAAV LEYLTAEILE LAGNAARDNK KTRIIPRHLQ LAIRNDEELN KLLGRVTIA QGGVLPNIQA VLLP UniProtKB: Histone H2A type 1-B/E |
-Macromolecule #4: Histone H2B type 1-J
| Macromolecule | Name: Histone H2B type 1-J / type: protein_or_peptide / ID: 4 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 10.334827 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SRKESYSIYV YKVLKQVHPD TGISSKAMGI MNSFVNDIFE RIAGEASRLA HYNKRSTITS REIQTAVRLL LPGELAKHAV SEGTKAVTK YTSA UniProtKB: Histone H2B type 1-J |
-Macromolecule #7: Cyclic GMP-AMP synthase
| Macromolecule | Name: Cyclic GMP-AMP synthase / type: protein_or_peptide / ID: 7 / Number of copies: 2 / Enantiomer: LEVO / EC number: cyclic GMP-AMP synthase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 42.75927 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DAAPGASKLR AVLEKLKLSR DDISTAAGMV KGVVDHLLLR LKCDSAFRGV GLLNTGSYYE HVKISAPNEF DVMFKLEVPR IQLEEYSNT RAYYFVKFKR NPKENPLSQF LEGEILSASK MLSKFRKIIK EEINDIKDTD VIMKRKRGGS PAVTLLISEK I SVDITLAL ...String: DAAPGASKLR AVLEKLKLSR DDISTAAGMV KGVVDHLLLR LKCDSAFRGV GLLNTGSYYE HVKISAPNEF DVMFKLEVPR IQLEEYSNT RAYYFVKFKR NPKENPLSQF LEGEILSASK MLSKFRKIIK EEINDIKDTD VIMKRKRGGS PAVTLLISEK I SVDITLAL ESKSSWPAST QEGLRIQNWL SAKVRKQLRL KPFYLVPKHA KEGNGFQEET WRLSFSHIEK EILNNHGKSK TC CENKEEK CCRKDCLKLM KYLLEQLKER FKDKKHLDKF SSYHVKTAFF HVCTQNPQDS QWDRKDLGLC FDNCVTYFLQ CLR TEKLEN YFIPEFNLFS SNLIDKRSKE FLTKQIEYER NNEFPVFDEF UniProtKB: Cyclic GMP-AMP synthase |
-Macromolecule #5: DNA (147-MER)
| Macromolecule | Name: DNA (147-MER) / type: dna / ID: 5 / Number of copies: 2 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.604047 KDa |
| Sequence | String: (DA)(DC)(DA)(DG)(DG)(DA)(DT)(DG)(DT)(DA) (DT)(DA)(DT)(DA)(DT)(DC)(DT)(DG)(DA)(DC) (DA)(DC)(DG)(DT)(DG)(DC)(DC)(DT)(DG) (DG)(DA)(DG)(DA)(DC)(DT)(DA)(DG)(DG)(DG) (DA) (DG)(DT)(DA)(DA)(DT)(DC) ...String: (DA)(DC)(DA)(DG)(DG)(DA)(DT)(DG)(DT)(DA) (DT)(DA)(DT)(DA)(DT)(DC)(DT)(DG)(DA)(DC) (DA)(DC)(DG)(DT)(DG)(DC)(DC)(DT)(DG) (DG)(DA)(DG)(DA)(DC)(DT)(DA)(DG)(DG)(DG) (DA) (DG)(DT)(DA)(DA)(DT)(DC)(DC)(DC) (DC)(DT)(DT)(DG)(DG)(DC)(DG)(DG)(DT)(DT) (DA)(DA) (DA)(DA)(DC)(DG)(DC)(DG)(DG) (DG)(DG)(DG)(DA)(DC)(DA)(DG)(DC)(DG)(DC) (DG)(DT)(DA) (DC)(DG)(DT)(DG)(DC)(DG) (DT)(DT)(DT)(DA)(DA)(DG)(DC)(DG)(DG)(DT) (DG)(DC)(DT)(DA) (DG)(DA)(DG)(DC)(DT) (DG)(DT)(DC)(DT)(DA)(DC)(DG)(DA)(DC)(DC) (DA)(DA)(DT)(DT)(DG) (DA)(DG)(DC)(DG) (DG)(DC)(DC)(DT)(DC)(DG)(DG)(DC)(DA)(DC) (DC)(DG)(DG)(DG)(DA)(DT) (DT)(DC)(DT) (DC)(DC)(DA)(DG) |
-Macromolecule #6: DNA (147-MER)
| Macromolecule | Name: DNA (147-MER) / type: dna / ID: 6 / Number of copies: 2 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.145754 KDa |
| Sequence | String: (DC)(DT)(DG)(DG)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA) (DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA)(DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA) (DG) (DA)(DC)(DA)(DG)(DC)(DT) ...String: (DC)(DT)(DG)(DG)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA) (DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA)(DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA) (DG) (DA)(DC)(DA)(DG)(DC)(DT)(DC)(DT) (DA)(DG)(DC)(DA)(DC)(DC)(DG)(DC)(DT)(DT) (DA)(DA) (DA)(DC)(DG)(DC)(DA)(DC)(DG) (DT)(DA)(DC)(DG)(DC)(DG)(DC)(DT)(DG)(DT) (DC)(DC)(DC) (DC)(DC)(DG)(DC)(DG)(DT) (DT)(DT)(DT)(DA)(DA)(DC)(DC)(DG)(DC)(DC) (DA)(DA)(DG)(DG) (DG)(DG)(DA)(DT)(DT) (DA)(DC)(DT)(DC)(DC)(DC)(DT)(DA)(DG)(DT) (DC)(DT)(DC)(DC)(DA) (DG)(DG)(DC)(DA) (DC)(DG)(DT)(DG)(DT)(DC)(DA)(DG)(DA)(DT) (DA)(DT)(DA)(DT)(DA)(DC) (DA)(DT)(DC) (DC)(DT)(DG)(DT) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)