+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the TUG891 bound GPR120-Giq complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / GPR120 / complex / fatty acid hormones / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of somatostatin secretion / ghrelin secretion / positive regulation of glucagon secretion / Free fatty acid receptors / regulation of D-glucose transmembrane transport / taste receptor activity / Fatty Acids bound to GPR40 (FFAR1) regulate insulin secretion / Acetylcholine regulates insulin secretion / PLC beta mediated events / phospholipase C-activating dopamine receptor signaling pathway ...negative regulation of somatostatin secretion / ghrelin secretion / positive regulation of glucagon secretion / Free fatty acid receptors / regulation of D-glucose transmembrane transport / taste receptor activity / Fatty Acids bound to GPR40 (FFAR1) regulate insulin secretion / Acetylcholine regulates insulin secretion / PLC beta mediated events / phospholipase C-activating dopamine receptor signaling pathway / entrainment of circadian clock / regulation of platelet activation / hormone secretion / phototransduction, visible light / arrestin family protein binding / regulation of canonical Wnt signaling pathway / glutamate receptor signaling pathway / ciliary membrane / negative regulation of cytokine production / negative regulation of interleukin-1 beta production / action potential / endocytic vesicle / white fat cell differentiation / photoreceptor outer segment / T cell migration / D2 dopamine receptor binding / Adenylate cyclase inhibitory pathway / positive regulation of protein localization to cell cortex / positive regulation of osteoblast differentiation / regulation of cAMP-mediated signaling / brown fat cell differentiation / G protein-coupled serotonin receptor binding / cellular response to forskolin / cellular response to hormone stimulus / regulation of mitotic spindle organization / positive regulation of brown fat cell differentiation / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / GTPase activator activity / fatty acid binding / Regulation of insulin secretion / G protein-coupled receptor binding / G protein-coupled receptor activity / negative regulation of protein kinase activity / peptide binding / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / Activation of the phototransduction cascade / adenylate cyclase-activating G protein-coupled receptor signaling pathway / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / cilium / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / response to peptide hormone / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / negative regulation of inflammatory response / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / sensory perception of taste / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / GDP binding / cellular response to prostaglandin E stimulus / blood coagulation / Inactivation, recovery and regulation of the phototransduction cascade / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / GTPase binding / positive regulation of cold-induced thermogenesis / retina development in camera-type eye / Ca2+ pathway / cell cortex / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cytosolic calcium ion concentration / midbody / G alpha (i) signalling events / fibroblast proliferation Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.64 Å | |||||||||
 Authors Authors | Mao C / Xiao P / Tao X / Qin J / He Q / Zhang C / Yu X / Zhang Y / Sun J | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2023 Journal: Science / Year: 2023Title: Unsaturated bond recognition leads to biased signal in a fatty acid receptor. Authors: Chunyou Mao / Peng Xiao / Xiao-Na Tao / Jiao Qin / Qing-Tao He / Chao Zhang / Sheng-Chao Guo / Ya-Qin Du / Li-Nan Chen / Dan-Dan Shen / Zhi-Shuai Yang / Han-Qiong Zhang / Shen-Ming Huang / ...Authors: Chunyou Mao / Peng Xiao / Xiao-Na Tao / Jiao Qin / Qing-Tao He / Chao Zhang / Sheng-Chao Guo / Ya-Qin Du / Li-Nan Chen / Dan-Dan Shen / Zhi-Shuai Yang / Han-Qiong Zhang / Shen-Ming Huang / Yong-Hao He / Jie Cheng / Ya-Ni Zhong / Pan Shang / Jun Chen / Dao-Lai Zhang / Qian-Lang Wang / Mei-Xia Liu / Guo-Yu Li / Yongyuan Guo / H Eric Xu / Chuanxin Wang / Cheng Zhang / Shiqing Feng / Xiao Yu / Yan Zhang / Jin-Peng Sun /  Abstract: Individual free fatty acids (FAs) play important roles in metabolic homeostasis, many through engagement with more than 40G protein-coupled receptors. Searching for receptors to sense beneficial ...Individual free fatty acids (FAs) play important roles in metabolic homeostasis, many through engagement with more than 40G protein-coupled receptors. Searching for receptors to sense beneficial omega-3 FAs of fish oil enabled the identification of GPR120, which is involved in a spectrum of metabolic diseases. Here, we report six cryo-electron microscopy structures of GPR120 in complex with FA hormones or TUG891 and G or G trimers. Aromatic residues inside the GPR120 ligand pocket were responsible for recognizing different double-bond positions of these FAs and connect ligand recognition to distinct effector coupling. We also investigated synthetic ligand selectivity and the structural basis of missense single-nucleotide polymorphisms. We reveal how GPR120 differentiates rigid double bonds and flexible single bonds. The knowledge gleaned here may facilitate rational drug design targeting to GPR120. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29736.map.gz emd_29736.map.gz | 882.7 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29736-v30.xml emd-29736-v30.xml emd-29736.xml emd-29736.xml | 17.5 KB 17.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_29736.png emd_29736.png | 81.7 KB | ||
| Filedesc metadata |  emd-29736.cif.gz emd-29736.cif.gz | 6.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29736 http://ftp.pdbj.org/pub/emdb/structures/EMD-29736 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29736 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29736 | HTTPS FTP |
-Validation report
| Summary document |  emd_29736_validation.pdf.gz emd_29736_validation.pdf.gz | 357.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29736_full_validation.pdf.gz emd_29736_full_validation.pdf.gz | 356.7 KB | Display | |
| Data in XML |  emd_29736_validation.xml.gz emd_29736_validation.xml.gz | 5.6 KB | Display | |
| Data in CIF |  emd_29736_validation.cif.gz emd_29736_validation.cif.gz | 6.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29736 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29736 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29736 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29736 | HTTPS FTP |
-Related structure data
| Related structure data |  8g59MC  8id3C  8id4C  8id6C  8id8C  8id9C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29736.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29736.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.014 Å | ||||||||||||||||||||||||||||||||||||
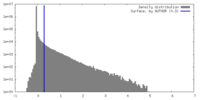
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Cryo-EM structure of the TUG891 bound GPR120-Giq complex
| Entire | Name: Cryo-EM structure of the TUG891 bound GPR120-Giq complex |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of the TUG891 bound GPR120-Giq complex
| Supramolecule | Name: Cryo-EM structure of the TUG891 bound GPR120-Giq complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.285734 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLII WDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR FLDDNQIVTS S GDTTCALW ...String: SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLII WDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR FLDDNQIVTS S GDTTCALW DIETGQQTTT FTGHTGDVMS LSLAPDTRLF VSGACDASAK LWDVREGMCR QTFTGHESDI NAICFFPNGN AF ATGSDDA TCRLFDLRAD QELMTYSHDN IICGITSVSF SKSGRLLLAG YDDFNCNVWD ALKADRAGVL AGHDNRVSCL GVT DDGMAV ATGSWDSFLK IWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #3: scFv16
| Macromolecule | Name: scFv16 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 30.363043 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFAVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSAG G GGSGGGGS ...String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFAVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSAG G GGSGGGGS GGGGSADIVM TQATSSVPVT PGESVSISCR SSKSLLHSNG NTYLYWFLQR PGQSPQLLIY RMSNLASGVP DR FSGSGSG TAFTLTISRL EAEDVGVYYC MQHLEYPLTF GAGTKLEL |
-Macromolecule #4: Guanine nucleotide-binding protein G(q) subunit alpha
| Macromolecule | Name: Guanine nucleotide-binding protein G(q) subunit alpha / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.555199 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKSTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKSTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTTGIVETH FTFKDLHFKM FDVGAQRSER KKWIHCFEGV TAIIFCVALS DYDLVLAEDE EM NRMHESM KLFDSICNNK WFTDTSIILF LNKKDLFEEK IKKSPLTICY PEYAGSNTYE EAAAYIQCQF EDLNKRKDTK EIY THFTCS TDTENIRFVF AAVKDTILQL NLKEYNLV UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1, Guanine nucleotide-binding protein G(q) subunit alpha |
-Macromolecule #5: Free fatty acid receptor 4
| Macromolecule | Name: Free fatty acid receptor 4 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.533211 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSPECARAAG DAPLRSLEQA NRTRFPFFSD VKGDHRLVLA AVETTVLVLI FAVSLLGNVC ALVLVARRRR RGATACLVLN LFCADLLFI SAIPLVLAVR WTEAWLLGPV ACHLLFYVMT LSGSVTILTL AAVSLERMVC IVHLQRGVRG PGRRARAVLL A LIWGYSAV ...String: MSPECARAAG DAPLRSLEQA NRTRFPFFSD VKGDHRLVLA AVETTVLVLI FAVSLLGNVC ALVLVARRRR RGATACLVLN LFCADLLFI SAIPLVLAVR WTEAWLLGPV ACHLLFYVMT LSGSVTILTL AAVSLERMVC IVHLQRGVRG PGRRARAVLL A LIWGYSAV AALPLCVFFR VVPQRLPGAD QEISICTLIW PTIPGEISWD VSFVTLNFLV PGLVIVISYS KILQITKASR KR LTVSLAY SESHQIRVSQ QDFRLFRTLF LLMVSFFIMW SPIIITILLI LIQNFKQDLV IWPSLFFWVV AFTFANSALN PIL YNMTLC RNEWKKIFCC FWFPEKGAIL TDTSVKRNDL SIISG UniProtKB: Free fatty acid receptor 4 |
-Macromolecule #6: 3-{4-[(4-fluoro-4'-methyl[1,1'-biphenyl]-2-yl)methoxy]phenyl}prop...
| Macromolecule | Name: 3-{4-[(4-fluoro-4'-methyl[1,1'-biphenyl]-2-yl)methoxy]phenyl}propanoic acid type: ligand / ID: 6 / Number of copies: 1 / Formula: YN9 |
|---|---|
| Molecular weight | Theoretical: 364.409 Da |
| Chemical component information | 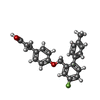 ChemComp-YN9: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.64 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 303739 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller











































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)