[English] 日本語
 Yorodumi
Yorodumi- EMDB-29489: Dimeric form of HIV-1 Vif in complex with human CBF-beta, ELOB, E... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Dimeric form of HIV-1 Vif in complex with human CBF-beta, ELOB, ELOC, and CUL5 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | virus-host protein complex / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationRUNX3 regulates RUNX1-mediated transcription / RUNX1 regulates transcription of genes involved in BCR signaling / RUNX1 regulates transcription of genes involved in interleukin signaling / RUNX2 regulates bone development / core-binding factor complex / RUNX1 regulates expression of components of tight junctions / positive regulation of CD8-positive, alpha-beta T cell differentiation / RUNX2 regulates chondrocyte maturation / negative regulation of CD4-positive, alpha-beta T cell differentiation / RUNX1 and FOXP3 control the development of regulatory T lymphocytes (Tregs) ...RUNX3 regulates RUNX1-mediated transcription / RUNX1 regulates transcription of genes involved in BCR signaling / RUNX1 regulates transcription of genes involved in interleukin signaling / RUNX2 regulates bone development / core-binding factor complex / RUNX1 regulates expression of components of tight junctions / positive regulation of CD8-positive, alpha-beta T cell differentiation / RUNX2 regulates chondrocyte maturation / negative regulation of CD4-positive, alpha-beta T cell differentiation / RUNX1 and FOXP3 control the development of regulatory T lymphocytes (Tregs) / lymphocyte differentiation / ERBB2 signaling pathway / RUNX2 regulates genes involved in cell migration / Transcriptional regulation by RUNX2 / RUNX2 regulates genes involved in differentiation of myeloid cells / RUNX1 regulates transcription of genes involved in differentiation of keratinocytes / reelin-mediated signaling pathway / RUNX3 Regulates Immune Response and Cell Migration / myeloid cell differentiation / regulation of neuron migration / definitive hemopoiesis / target-directed miRNA degradation / elongin complex / protein K11-linked ubiquitination / RUNX1 regulates transcription of genes involved in differentiation of myeloid cells / Regulation of RUNX1 Expression and Activity / VCB complex / Cul5-RING ubiquitin ligase complex / SCF ubiquitin ligase complex / ubiquitin-dependent protein catabolic process via the C-end degron rule pathway / RUNX1 regulates transcription of genes involved in WNT signaling / RUNX1 regulates estrogen receptor mediated transcription / Cul2-RING ubiquitin ligase complex / SCF-dependent proteasomal ubiquitin-dependent protein catabolic process / RUNX2 regulates osteoblast differentiation / ubiquitin ligase complex scaffold activity / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / RUNX3 regulates p14-ARF / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / Tat-mediated elongation of the HIV-1 transcript / site of DNA damage / Formation of HIV-1 elongation complex containing HIV-1 Tat / Formation of HIV elongation complex in the absence of HIV Tat / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / viral life cycle / cell maturation / RNA Polymerase II Pre-transcription Events / intrinsic apoptotic signaling pathway / transcription corepressor binding / TP53 Regulates Transcription of DNA Repair Genes / transcription initiation at RNA polymerase II promoter / transcription elongation by RNA polymerase II / G1/S transition of mitotic cell cycle / Regulation of RUNX3 expression and activity / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / Vif-mediated degradation of APOBEC3G / Inactivation of CSF3 (G-CSF) signaling / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / Evasion by RSV of host interferon responses / virion component / calcium channel activity / Downregulation of ERBB2 signaling / Regulation of expression of SLITs and ROBOs / Transcriptional regulation of granulopoiesis / protein polyubiquitination / Regulation of RUNX2 expression and activity / osteoblast differentiation / ubiquitin-protein transferase activity / Antigen processing: Ubiquitination & Proteasome degradation / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / signaling receptor activity / RUNX1 regulates transcription of genes involved in differentiation of HSCs / Neddylation / protein-containing complex assembly / protein-macromolecule adaptor activity / Estrogen-dependent gene expression / sequence-specific DNA binding / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process / transcription by RNA polymerase II / host cell cytoplasm / transcription coactivator activity / protein ubiquitination / ubiquitin protein ligase binding / regulation of transcription by RNA polymerase II / host cell plasma membrane / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / RNA binding / nucleoplasm / membrane / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | |||||||||
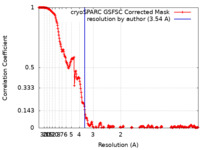
| Method | single particle reconstruction / cryo EM / Resolution: 3.54 Å | |||||||||
 Authors Authors | Ito F / Alvarez-Cabrera AL / Zhou ZH / Chen XS | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis of HIV-1 Vif-mediated E3 ligase targeting of host APOBEC3H. Authors: Fumiaki Ito / Ana L Alvarez-Cabrera / Kyumin Kim / Z Hong Zhou / Xiaojiang S Chen /  Abstract: Human APOBEC3 (A3) cytidine deaminases are antiviral factors that are particularly potent against retroviruses. As a countermeasure, HIV-1 uses a viral infectivity factor (Vif) to target specific ...Human APOBEC3 (A3) cytidine deaminases are antiviral factors that are particularly potent against retroviruses. As a countermeasure, HIV-1 uses a viral infectivity factor (Vif) to target specific human A3s for proteasomal degradation. Vif recruits cellular transcription cofactor CBF-β and Cullin-5 (CUL5) RING E3 ubiquitin ligase to bind different A3s distinctively, but how this is accomplished remains unclear in the absence of the atomic structure of the complex. Here, we present the cryo-EM structures of HIV-1 Vif in complex with human A3H, CBF-β and components of CUL5 ubiquitin ligase (CUL5, ELOB, and ELOC). Vif nucleates the entire complex by directly binding four human proteins, A3H, CBF-β, CUL5, and ELOC. The structures reveal a large interface area between A3H and Vif, primarily mediated by an α-helical side of A3H and a five-stranded β-sheet of Vif. This A3H-Vif interface unveils the basis for sensitivity-modulating polymorphism of both proteins, including a previously reported gain-of-function mutation in Vif isolated from HIV/AIDS patients. Our structural and functional results provide insights into the remarkable interplay between HIV and humans and would inform development efforts for anti-HIV therapeutics. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29489.map.gz emd_29489.map.gz | 1.3 GB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29489-v30.xml emd-29489-v30.xml emd-29489.xml emd-29489.xml | 23.6 KB 23.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29489_fsc.xml emd_29489_fsc.xml | 24 KB | Display |  FSC data file FSC data file |
| Images |  emd_29489.png emd_29489.png | 98.4 KB | ||
| Filedesc metadata |  emd-29489.cif.gz emd-29489.cif.gz | 6.8 KB | ||
| Others |  emd_29489_half_map_1.map.gz emd_29489_half_map_1.map.gz emd_29489_half_map_2.map.gz emd_29489_half_map_2.map.gz | 1.3 GB 1.3 GB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29489 http://ftp.pdbj.org/pub/emdb/structures/EMD-29489 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29489 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29489 | HTTPS FTP |
-Related structure data
| Related structure data |  8fvjMC  8fviC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29489.map.gz / Format: CCP4 / Size: 1.4 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29489.map.gz / Format: CCP4 / Size: 1.4 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.51 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_29489_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_29489_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Dimeric form of HIV-1 Vif in complex with human CBF-beta, ELOB, E...
| Entire | Name: Dimeric form of HIV-1 Vif in complex with human CBF-beta, ELOB, ELOC, and CUL5 |
|---|---|
| Components |
|
-Supramolecule #1: Dimeric form of HIV-1 Vif in complex with human CBF-beta, ELOB, E...
| Supramolecule | Name: Dimeric form of HIV-1 Vif in complex with human CBF-beta, ELOB, ELOC, and CUL5 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 198 KDa |
-Macromolecule #1: Core-binding factor subunit beta
| Macromolecule | Name: Core-binding factor subunit beta / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 18.643814 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPRVVPDQRS KFENEEFFRK LSRECEIKYT GFRDRPHEER QARFQNACRD GRSEIAFVAT GTNLSLQFFP ASWQGEQRQT PSREYVDLE REAGKVYLKA PMILNGVCVI WKGWIDLQRL DGMGCLEFDE ERAQQEDALA QQAFEEARRR TREFEDRD UniProtKB: Core-binding factor subunit beta |
-Macromolecule #2: Virion infectivity factor
| Macromolecule | Name: Virion infectivity factor / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 / Strain: pNL4-3 Human immunodeficiency virus 1 / Strain: pNL4-3 |
| Molecular weight | Theoretical: 21.160451 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPMENRWQVM IVWQVDRMRI NTWKRLVKHH MYISRKAKDW FYRHHYESTH PKISSEVHIP LGDAKLVITT YWGLHTGERD WHLGQGVSI EWRKKRYSTQ VDPDLADQLI HLHYFDCFSE SAIRNTILGR IVSPRCEYQA GHNKVGSLQY LALAALIKPK Q IKPPLPSV RKLTEDRWNK UniProtKB: Virion infectivity factor |
-Macromolecule #3: Elongin-B
| Macromolecule | Name: Elongin-B / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.48803 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDVFLMIRRH KTTIFTDAKE SSTVFELKRI VEGILKRPPD EQRLYKDDQL LDDGKTLGEC GFTSQTARPQ APATVGLAFR ADDTFEALC IEPFSSPPEL PDV UniProtKB: Elongin-B |
-Macromolecule #4: Elongin-C
| Macromolecule | Name: Elongin-C / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 10.84342 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MYVKLISSDG HEFIVKREHA LTSGTIKAML SGPGQFAENE TNEVNFREIP SHVLSKVCMY FTYKVRYTNS STEIPEFPIA PEIALELLM AANFLDC UniProtKB: Elongin-C |
-Macromolecule #5: Cullin-5
| Macromolecule | Name: Cullin-5 / type: protein_or_peptide / ID: 5 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 36.61398 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPAGSSLQFE DKWDFMRPIV LKLLRQESVT KQQWFDLFSD VHAVCLWDDK GPAKIHQALK EDILEFIKQA QARVLSHQDD TALLKAYIV EWRKFFTQCD ILPKPFCQLE ITLMGKQGSN KKSNVEDSIV RKLMLDTWNE SIFSNIKNRL QDSAMKLVHA E RLGEAFDS ...String: GPAGSSLQFE DKWDFMRPIV LKLLRQESVT KQQWFDLFSD VHAVCLWDDK GPAKIHQALK EDILEFIKQA QARVLSHQDD TALLKAYIV EWRKFFTQCD ILPKPFCQLE ITLMGKQGSN KKSNVEDSIV RKLMLDTWNE SIFSNIKNRL QDSAMKLVHA E RLGEAFDS QLVIGVRESY VNLCSNPEDK LQIYRDNFEK AYLDSTERFY RTQAPSYLQQ NGVQNYMKYA DAKLKEEEKR AL RYLETRR ECNSVEALME CCVNALVTSF KETILAECQG MIKRNETEKL HLMFSLMDKV PNGIEPMLKD LEEHIIS UniProtKB: Cullin-5 |
-Macromolecule #6: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 6 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 14725 / Average exposure time: 3.5 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 165000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)