[English] 日本語
 Yorodumi
Yorodumi- EMDB-29488: Human APOBEC3H bound to HIV-1 Vif in complex with CBF-beta, ELOB,... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human APOBEC3H bound to HIV-1 Vif in complex with CBF-beta, ELOB, ELOC, and CUL5 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | virus-host protein complex / ANTIVIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationRUNX3 regulates RUNX1-mediated transcription / RUNX1 regulates transcription of genes involved in BCR signaling / mRNA Editing: C to U Conversion / Formation of the Editosome / RUNX1 regulates transcription of genes involved in interleukin signaling / RUNX2 regulates bone development / core-binding factor complex / RUNX1 regulates expression of components of tight junctions / positive regulation of CD8-positive, alpha-beta T cell differentiation / RUNX2 regulates chondrocyte maturation ...RUNX3 regulates RUNX1-mediated transcription / RUNX1 regulates transcription of genes involved in BCR signaling / mRNA Editing: C to U Conversion / Formation of the Editosome / RUNX1 regulates transcription of genes involved in interleukin signaling / RUNX2 regulates bone development / core-binding factor complex / RUNX1 regulates expression of components of tight junctions / positive regulation of CD8-positive, alpha-beta T cell differentiation / RUNX2 regulates chondrocyte maturation / single-stranded DNA cytosine deaminase / negative regulation of CD4-positive, alpha-beta T cell differentiation / negative regulation of single stranded viral RNA replication via double stranded DNA intermediate / DNA cytosine deamination / cytidine to uridine editing / host-mediated suppression of viral genome replication / clearance of foreign intracellular DNA / RUNX1 and FOXP3 control the development of regulatory T lymphocytes (Tregs) / cytidine deaminase activity / lymphocyte differentiation / RUNX2 regulates genes involved in cell migration / Transcriptional regulation by RUNX2 / RUNX2 regulates genes involved in differentiation of myeloid cells / RUNX1 regulates transcription of genes involved in differentiation of keratinocytes / transposable element silencing / RUNX3 Regulates Immune Response and Cell Migration / myeloid cell differentiation / cullin-RING ubiquitin ligase complex / definitive hemopoiesis / target-directed miRNA degradation / elongin complex / RUNX1 regulates transcription of genes involved in differentiation of myeloid cells / Regulation of RUNX1 Expression and Activity / VCB complex / Cul5-RING ubiquitin ligase complex / ubiquitin-dependent protein catabolic process via the C-end degron rule pathway / RUNX1 regulates transcription of genes involved in WNT signaling / RUNX1 regulates estrogen receptor mediated transcription / Cul2-RING ubiquitin ligase complex / RUNX2 regulates osteoblast differentiation / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / RUNX3 regulates p14-ARF / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / Formation of HIV elongation complex in the absence of HIV Tat / proteasomal protein catabolic process / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / viral life cycle / cell maturation / RNA Polymerase II Pre-transcription Events / transcription corepressor binding / TP53 Regulates Transcription of DNA Repair Genes / transcription initiation at RNA polymerase II promoter / transcription elongation by RNA polymerase II / P-body / Regulation of RUNX3 expression and activity / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / Vif-mediated degradation of APOBEC3G / Inactivation of CSF3 (G-CSF) signaling / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / Evasion by RSV of host interferon responses / virion component / Regulation of expression of SLITs and ROBOs / Transcriptional regulation of granulopoiesis / protein polyubiquitination / Regulation of RUNX2 expression and activity / osteoblast differentiation / Antigen processing: Ubiquitination & Proteasome degradation / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / RUNX1 regulates transcription of genes involved in differentiation of HSCs / Neddylation / protein-containing complex assembly / protein-macromolecule adaptor activity / defense response to virus / Estrogen-dependent gene expression / sequence-specific DNA binding / ubiquitin-dependent protein catabolic process / transcription by RNA polymerase II / host cell cytoplasm / transcription coactivator activity / protein ubiquitination / innate immune response / ubiquitin protein ligase binding / regulation of transcription by RNA polymerase II / host cell plasma membrane / negative regulation of transcription by RNA polymerase II / signal transduction / positive regulation of transcription by RNA polymerase II / RNA binding / zinc ion binding / nucleoplasm / membrane / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   Human immunodeficiency virus 1 / Human immunodeficiency virus 1 /  | |||||||||
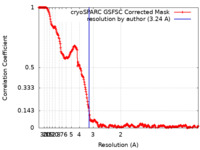
| Method | single particle reconstruction / cryo EM / Resolution: 3.24 Å | |||||||||
 Authors Authors | Ito F / Alvarez-Cabrera AL / Zhou ZH / Chen XS | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis of HIV-1 Vif-mediated E3 ligase targeting of host APOBEC3H. Authors: Fumiaki Ito / Ana L Alvarez-Cabrera / Kyumin Kim / Z Hong Zhou / Xiaojiang S Chen /  Abstract: Human APOBEC3 (A3) cytidine deaminases are antiviral factors that are particularly potent against retroviruses. As a countermeasure, HIV-1 uses a viral infectivity factor (Vif) to target specific ...Human APOBEC3 (A3) cytidine deaminases are antiviral factors that are particularly potent against retroviruses. As a countermeasure, HIV-1 uses a viral infectivity factor (Vif) to target specific human A3s for proteasomal degradation. Vif recruits cellular transcription cofactor CBF-β and Cullin-5 (CUL5) RING E3 ubiquitin ligase to bind different A3s distinctively, but how this is accomplished remains unclear in the absence of the atomic structure of the complex. Here, we present the cryo-EM structures of HIV-1 Vif in complex with human A3H, CBF-β and components of CUL5 ubiquitin ligase (CUL5, ELOB, and ELOC). Vif nucleates the entire complex by directly binding four human proteins, A3H, CBF-β, CUL5, and ELOC. The structures reveal a large interface area between A3H and Vif, primarily mediated by an α-helical side of A3H and a five-stranded β-sheet of Vif. This A3H-Vif interface unveils the basis for sensitivity-modulating polymorphism of both proteins, including a previously reported gain-of-function mutation in Vif isolated from HIV/AIDS patients. Our structural and functional results provide insights into the remarkable interplay between HIV and humans and would inform development efforts for anti-HIV therapeutics. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29488.map.gz emd_29488.map.gz | 1.3 GB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29488-v30.xml emd-29488-v30.xml emd-29488.xml emd-29488.xml | 27.2 KB 27.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29488_fsc.xml emd_29488_fsc.xml | 23.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_29488.png emd_29488.png | 86.1 KB | ||
| Filedesc metadata |  emd-29488.cif.gz emd-29488.cif.gz | 7.5 KB | ||
| Others |  emd_29488_half_map_1.map.gz emd_29488_half_map_1.map.gz emd_29488_half_map_2.map.gz emd_29488_half_map_2.map.gz | 1.3 GB 1.3 GB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29488 http://ftp.pdbj.org/pub/emdb/structures/EMD-29488 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29488 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29488 | HTTPS FTP |
-Related structure data
| Related structure data |  8fviMC  8fvjC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29488.map.gz / Format: CCP4 / Size: 1.4 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29488.map.gz / Format: CCP4 / Size: 1.4 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.51 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_29488_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_29488_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Hetero-hexameric complex of HIV-1 Vif and human APOBEC3H, CBF-bet...
+Supramolecule #1: Hetero-hexameric complex of HIV-1 Vif and human APOBEC3H, CBF-bet...
+Macromolecule #1: Core-binding factor subunit beta
+Macromolecule #2: Virion infectivity factor
+Macromolecule #3: DNA dC->dU-editing enzyme APOBEC-3H
+Macromolecule #6: Cullin 5
+Macromolecule #7: Elongin-B
+Macromolecule #8: Elongin-C
+Macromolecule #4: RNA(5'-R(AP*UP*UP*UP*UP*UP*UP*UP*UP*U)-3')
+Macromolecule #5: RNA(5'-R(*AP*AP*AP*AP*AP*AP*AP*AP*A)-3')
+Macromolecule #9: ZINC ION
+Macromolecule #10: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 14725 / Average exposure time: 3.5 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 165000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)