+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2828 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Mot1 in complex with TBP, NC2 and DNA | |||||||||
 Map data Map data | Negative Stain reconstruction of the Mot1 TBP NC2 complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Swi/Snf2 / chromatin remodelling / TBP / ATPase | |||||||||
| Biological species |  Encephalitozoon cuniculi (fungus) Encephalitozoon cuniculi (fungus) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 22.0 Å | |||||||||
 Authors Authors | Butryn A / Schuller JM / Stoehr G / Runge-Wollmann P / Foerster F / Auble DT / Hopfner K-P | |||||||||
 Citation Citation |  Journal: Elife / Year: 2015 Journal: Elife / Year: 2015Title: Structural basis for recognition and remodeling of the TBP:DNA:NC2 complex by Mot1. Authors: Agata Butryn / Jan M Schuller / Gabriele Stoehr / Petra Runge-Wollmann / Friedrich Förster / David T Auble / Karl-Peter Hopfner /   Abstract: Swi2/Snf2 ATPases remodel substrates such as nucleosomes and transcription complexes to control a wide range of DNA-associated processes, but detailed structural information on the ATP-dependent ...Swi2/Snf2 ATPases remodel substrates such as nucleosomes and transcription complexes to control a wide range of DNA-associated processes, but detailed structural information on the ATP-dependent remodeling reactions is largely absent. The single subunit remodeler Mot1 (modifier of transcription 1) dissociates TATA box-binding protein (TBP):DNA complexes, offering a useful system to address the structural mechanisms of Swi2/Snf2 ATPases. Here, we report the crystal structure of the N-terminal domain of Mot1 in complex with TBP, DNA, and the transcription regulator negative cofactor 2 (NC2). Our data show that Mot1 reduces DNA:NC2 interactions and unbends DNA as compared to the TBP:DNA:NC2 state, suggesting that Mot1 primes TBP:NC2 displacement in an ATP-independent manner. Electron microscopy and cross-linking data suggest that the Swi2/Snf2 domain of Mot1 associates with the upstream DNA and the histone fold of NC2, thereby revealing parallels to some nucleosome remodelers. This study provides a structural framework for how a Swi2/Snf2 ATPase interacts with its substrate DNA:protein complex. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2828.map.gz emd_2828.map.gz | 12 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2828-v30.xml emd-2828-v30.xml emd-2828.xml emd-2828.xml | 8 KB 8 KB | Display Display |  EMDB header EMDB header |
| Images |  EMD-2828.tif EMD-2828.tif emd_2828.png emd_2828.png | 85.8 KB 136 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2828 http://ftp.pdbj.org/pub/emdb/structures/EMD-2828 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2828 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2828 | HTTPS FTP |
-Validation report
| Summary document |  emd_2828_validation.pdf.gz emd_2828_validation.pdf.gz | 223.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_2828_full_validation.pdf.gz emd_2828_full_validation.pdf.gz | 222.7 KB | Display | |
| Data in XML |  emd_2828_validation.xml.gz emd_2828_validation.xml.gz | 5.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2828 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2828 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2828 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2828 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_2828.map.gz / Format: CCP4 / Size: 12.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2828.map.gz / Format: CCP4 / Size: 12.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Negative Stain reconstruction of the Mot1 TBP NC2 complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.61 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
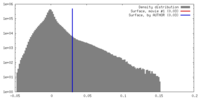
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Mot1 in complex with TBP, NC2a, NC2b and DNA
| Entire | Name: Mot1 in complex with TBP, NC2a, NC2b and DNA |
|---|---|
| Components |
|
-Supramolecule #1000: Mot1 in complex with TBP, NC2a, NC2b and DNA
| Supramolecule | Name: Mot1 in complex with TBP, NC2a, NC2b and DNA / type: sample / ID: 1000 Details: only the C-terminal part of Mot1 was used in the construct Number unique components: 5 |
|---|
-Macromolecule #1: Mot1
| Macromolecule | Name: Mot1 / type: protein_or_peptide / ID: 1 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Encephalitozoon cuniculi (fungus) Encephalitozoon cuniculi (fungus) |
| Recombinant expression | Recombinant cell: Sf9 |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.05 mg/mL |
|---|---|
| Buffer | pH: 6.5 / Details: 20 mM MES pH, 60 mM KCl, 5 mM MgCl2 and 2 mM DTT |
| Staining | Type: NEGATIVE Details: Grids with adsorbed protein floated on 1% w/v uranyl acetate for 20 seconds. |
| Grid | Details: 200 mesh carbon support grid for negative stain |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG |
|---|---|
| Date | Jan 28, 2014 |
| Image recording | Category: CCD / Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Number real images: 144 / Average electron dose: 25 e/Å2 |
| Electron beam | Acceleration voltage: 160 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 62000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC |
- Image processing
Image processing
| Details | The particles were manually selected using e2boxer |
|---|---|
| CTF correction | Details: Micrograph level |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 22.0 Å / Resolution method: OTHER / Software - Name: XMIPP / Number images used: 8192 |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)