[English] 日本語
 Yorodumi
Yorodumi- EMDB-2798: single-particle cryo reconstruction of the Large Ribosomal subuni... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2798 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | single-particle cryo reconstruction of the Large Ribosomal subunit-associated protein Quality Control (RQC) complex in the context of Tae2 deletion | |||||||||
 Map data Map data | reconstruction of RQC complex in the context of Tae2 protein deletion | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RING domain E3 ubiquitin ligase / translational surveillance / protein quality control / cryo-EM / listerin/Ltn1 / Tae2/Nemf | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 11.2 Å | |||||||||
 Authors Authors | Lyumkis D / Oliveira dos Passos D / Tahara EB / Webb K / Bennett EJ / Vinterbo S / Potter CS / Carragher B / Joazeiro CAP | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2014 Journal: Proc Natl Acad Sci U S A / Year: 2014Title: Structural basis for translational surveillance by the large ribosomal subunit-associated protein quality control complex. Authors: Dmitry Lyumkis / Dario Oliveira dos Passos / Erich B Tahara / Kristofor Webb / Eric J Bennett / Staal Vinterbo / Clinton S Potter / Bridget Carragher / Claudio A P Joazeiro /  Abstract: All organisms have evolved mechanisms to manage the stalling of ribosomes upon translation of aberrant mRNA. In eukaryotes, the large ribosomal subunit-associated quality control complex (RQC), ...All organisms have evolved mechanisms to manage the stalling of ribosomes upon translation of aberrant mRNA. In eukaryotes, the large ribosomal subunit-associated quality control complex (RQC), composed of the listerin/Ltn1 E3 ubiquitin ligase and cofactors, mediates the ubiquitylation and extraction of ribosome-stalled nascent polypeptide chains for proteasomal degradation. How RQC recognizes stalled ribosomes and performs its functions has not been understood. Using single-particle cryoelectron microscopy, we have determined the structure of the RQC complex bound to stalled 60S ribosomal subunits. The structure establishes how Ltn1 associates with the large ribosomal subunit and properly positions its E3-catalytic RING domain to mediate nascent chain ubiquitylation. The structure also reveals that a distinguishing feature of stalled 60S particles is an exposed, nascent chain-conjugated tRNA, and that the Tae2 subunit of RQC, which facilitates Ltn1 binding, is responsible for selective recognition of stalled 60S subunits. RQC components are engaged in interactions across a large span of the 60S subunit surface, connecting the tRNA in the peptidyl transferase center to the distally located nascent chain tunnel exit. This work provides insights into a mechanism linking translation and protein degradation that targets defective proteins immediately after synthesis, while ignoring nascent chains in normally translating ribosomes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2798.map.gz emd_2798.map.gz | 6.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2798-v30.xml emd-2798-v30.xml emd-2798.xml emd-2798.xml | 10.6 KB 10.6 KB | Display Display |  EMDB header EMDB header |
| Images |  EMD-2798.png EMD-2798.png | 95.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2798 http://ftp.pdbj.org/pub/emdb/structures/EMD-2798 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2798 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2798 | HTTPS FTP |
-Validation report
| Summary document |  emd_2798_validation.pdf.gz emd_2798_validation.pdf.gz | 231.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_2798_full_validation.pdf.gz emd_2798_full_validation.pdf.gz | 230.6 KB | Display | |
| Data in XML |  emd_2798_validation.xml.gz emd_2798_validation.xml.gz | 5.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2798 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2798 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2798 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2798 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_2798.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2798.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | reconstruction of RQC complex in the context of Tae2 protein deletion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.38 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
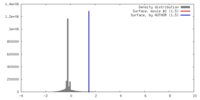
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Large 60S ribosomal subunit in complex with protein quality contr...
| Entire | Name: Large 60S ribosomal subunit in complex with protein quality control components, in the context of Tae2 protein deletion |
|---|---|
| Components |
|
-Supramolecule #1000: Large 60S ribosomal subunit in complex with protein quality contr...
| Supramolecule | Name: Large 60S ribosomal subunit in complex with protein quality control components, in the context of Tae2 protein deletion type: sample / ID: 1000 / Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 2.5 MDa |
-Supramolecule #1: Large 60S ribosomal subunit
| Supramolecule | Name: Large 60S ribosomal subunit / type: complex / ID: 1 / Recombinant expression: No / Ribosome-details: ribosome-eukaryote: LSU 60S |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 2.5 MDa |
-Macromolecule #1: Large ribosomal subunit-associated quality control complexlex
| Macromolecule | Name: Large ribosomal subunit-associated quality control complexlex type: protein_or_peptide / ID: 1 / Name.synonym: RQC / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL |
|---|---|
| Buffer | pH: 6.8 Details: 50 mM Hepes-KOH, 100 mM KOAc, 5 mM MgOAc, 1 mM EDTA, 2 mM DTT, 2x protease inhibitors, 0.1% Igepal CA-630 |
| Grid | Details: freshly plasma-cleaned holey carbon C-flat grid (Protochips) that had been overlaid with 2 nm thin carbon film |
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 93 K / Instrument: HOMEMADE PLUNGER Method: specimens were prepared for cryo-EM by applying 3 microliters of sample to a freshly plasma-cleaned holey carbon C-flat grid (Protochips) that had been overlaid with 2 nm thin carbon film, ...Method: specimens were prepared for cryo-EM by applying 3 microliters of sample to a freshly plasma-cleaned holey carbon C-flat grid (Protochips) that had been overlaid with 2 nm thin carbon film, allowing the sample to adsorb to the grid for 30 s, followed by blotting with filter paper and plunge freezing into liquid ethane using a manual cryoplunger in an ambient environment at 4 C. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Temperature | Average: 93 K |
| Alignment procedure | Legacy - Astigmatism: objective lens astigmatism was corrected by monitoring power spectra of continuously acquired images in leginon |
| Date | May 22, 2013 |
| Image recording | Category: CCD / Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Digitization - Sampling interval: 15.6 µm / Number real images: 2048 / Average electron dose: 32 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 92307 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 62000 |
| Sample stage | Specimen holder: 626 / Specimen holder model: GATAN LIQUID NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | see detailed methods in paper |
|---|---|
| CTF correction | Details: each particle |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 11.2 Å / Resolution method: OTHER / Software - Name: Frealign / Number images used: 37819 |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)