[English] 日本語
 Yorodumi
Yorodumi- EMDB-27736: RyR1 in presence of IpCa-T26E phosphomimetic and activating ligands -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | RyR1 in presence of IpCa-T26E phosphomimetic and activating ligands | |||||||||
 Map data Map data | RyR1 in presence of IpCa-T26E phosphomimetic and activating ligands | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ryanodine receptor / Ion channel / Snake toxin / Calcin / Complex / Membrane protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationATP-gated ion channel activity / : / negative regulation of calcium-mediated signaling / negative regulation of insulin secretion involved in cellular response to glucose stimulus / neuronal action potential propagation / negative regulation of release of sequestered calcium ion into cytosol / insulin secretion involved in cellular response to glucose stimulus / terminal cisterna / ryanodine-sensitive calcium-release channel activity / ryanodine receptor complex ...ATP-gated ion channel activity / : / negative regulation of calcium-mediated signaling / negative regulation of insulin secretion involved in cellular response to glucose stimulus / neuronal action potential propagation / negative regulation of release of sequestered calcium ion into cytosol / insulin secretion involved in cellular response to glucose stimulus / terminal cisterna / ryanodine-sensitive calcium-release channel activity / ryanodine receptor complex / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / CaM pathway / response to redox state / Cam-PDE 1 activation / ossification involved in bone maturation / Sodium/Calcium exchangers / Calmodulin induced events / negative regulation of heart rate / cellular response to caffeine / 'de novo' protein folding / Reduction of cytosolic Ca++ levels / Activation of Ca-permeable Kainate Receptor / CREB1 phosphorylation through the activation of CaMKII/CaMKK/CaMKIV cascasde / Loss of phosphorylation of MECP2 at T308 / CREB1 phosphorylation through the activation of Adenylate Cyclase / skin development / negative regulation of high voltage-gated calcium channel activity / PKA activation / CaMK IV-mediated phosphorylation of CREB / Glycogen breakdown (glycogenolysis) / CLEC7A (Dectin-1) induces NFAT activation / FK506 binding / Activation of RAC1 downstream of NMDARs / negative regulation of ryanodine-sensitive calcium-release channel activity / organelle localization by membrane tethering / mitochondrion-endoplasmic reticulum membrane tethering / autophagosome membrane docking / negative regulation of calcium ion export across plasma membrane / regulation of cardiac muscle cell action potential / organelle membrane / presynaptic endocytosis / regulation of cell communication by electrical coupling involved in cardiac conduction / Synthesis of IP3 and IP4 in the cytosol / Phase 0 - rapid depolarisation / intracellularly gated calcium channel activity / Negative regulation of NMDA receptor-mediated neuronal transmission / calcineurin-mediated signaling / smooth endoplasmic reticulum / Unblocking of NMDA receptors, glutamate binding and activation / outflow tract morphogenesis / RHO GTPases activate PAKs / regulation of ryanodine-sensitive calcium-release channel activity / Ion transport by P-type ATPases / Uptake and function of anthrax toxins / smooth muscle contraction / Long-term potentiation / protein phosphatase activator activity / Calcineurin activates NFAT / Regulation of MECP2 expression and activity / DARPP-32 events / Smooth Muscle Contraction / detection of calcium ion / regulation of cardiac muscle contraction / T cell proliferation / toxic substance binding / striated muscle contraction / catalytic complex / RHO GTPases activate IQGAPs / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / voltage-gated calcium channel activity / calcium channel inhibitor activity / Activation of AMPK downstream of NMDARs / presynaptic cytosol / skeletal muscle fiber development / cellular response to interferon-beta / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / Protein methylation / Ion homeostasis / eNOS activation / titin binding / Tetrahydrobiopterin (BH4) synthesis, recycling, salvage and regulation / release of sequestered calcium ion into cytosol / regulation of calcium-mediated signaling / voltage-gated potassium channel complex / FCERI mediated Ca+2 mobilization / calcium channel complex / sarcoplasmic reticulum membrane / substantia nigra development / regulation of heart rate / FCGR3A-mediated IL10 synthesis / cellular response to calcium ion / Ras activation upon Ca2+ influx through NMDA receptor / Antigen activates B Cell Receptor (BCR) leading to generation of second messengers / muscle contraction / calyx of Held / adenylate cyclase activator activity / protein serine/threonine kinase activator activity / VEGFR2 mediated cell proliferation / sarcomere / regulation of cytokinesis Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
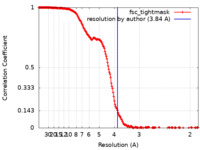
| Method | single particle reconstruction / cryo EM / Resolution: 3.84 Å | |||||||||
 Authors Authors | Haji-Ghassemi O / Van Petegm F | |||||||||
| Funding support |  Canada, 2 items Canada, 2 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2023 Journal: Sci Adv / Year: 2023Title: Cryo-EM analysis of scorpion toxin binding to Ryanodine Receptors reveals subconductance that is abolished by PKA phosphorylation. Authors: Omid Haji-Ghassemi / Yu Seby Chen / Kellie Woll / Georgina B Gurrola / Carmen R Valdivia / Wenxuan Cai / Songhua Li / Hector H Valdivia / Filip Van Petegem /     Abstract: Calcins are peptides from scorpion venom with the unique ability to cross cell membranes, gaining access to intracellular targets. Ryanodine Receptors (RyR) are intracellular ion channels that ...Calcins are peptides from scorpion venom with the unique ability to cross cell membranes, gaining access to intracellular targets. Ryanodine Receptors (RyR) are intracellular ion channels that control release of Ca from the endoplasmic and sarcoplasmic reticulum. Calcins target RyRs and induce long-lived subconductance states, whereby single-channel currents are decreased. We used cryo-electron microscopy to reveal the binding and structural effects of imperacalcin, showing that it opens the channel pore and causes large asymmetry throughout the cytosolic assembly of the tetrameric RyR. This also creates multiple extended ion conduction pathways beyond the transmembrane region, resulting in subconductance. Phosphorylation of imperacalcin by protein kinase A prevents its binding to RyR through direct steric hindrance, showing how posttranslational modifications made by the host organism can determine the fate of a natural toxin. The structure provides a direct template for developing calcin analogs that result in full channel block, with potential to treat RyR-related disorders. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27736.map.gz emd_27736.map.gz | 481.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27736-v30.xml emd-27736-v30.xml emd-27736.xml emd-27736.xml | 26.6 KB 26.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27736_fsc.xml emd_27736_fsc.xml | 19.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_27736.png emd_27736.png | 85.2 KB | ||
| Filedesc metadata |  emd-27736.cif.gz emd-27736.cif.gz | 10.1 KB | ||
| Others |  emd_27736_half_map_1.map.gz emd_27736_half_map_1.map.gz emd_27736_half_map_2.map.gz emd_27736_half_map_2.map.gz | 474.8 MB 474.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27736 http://ftp.pdbj.org/pub/emdb/structures/EMD-27736 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27736 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27736 | HTTPS FTP |
-Related structure data
| Related structure data |  8dveMC  8drpC  8dtbC  8dujC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27736.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27736.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RyR1 in presence of IpCa-T26E phosphomimetic and activating ligands | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.94 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: RyR1 in presence of IpCa-T26E phosphomimetic and activating ligands
| File | emd_27736_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RyR1 in presence of IpCa-T26E phosphomimetic and activating ligands | ||||||||||||
| Projections & Slices |
| ||||||||||||
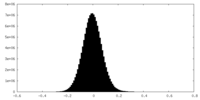
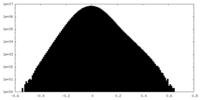
| Density Histograms |
-Half map: RyR1 in presence of IpCa-T26E phosphomimetic and activating ligands
| File | emd_27736_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RyR1 in presence of IpCa-T26E phosphomimetic and activating ligands | ||||||||||||
| Projections & Slices |
| ||||||||||||
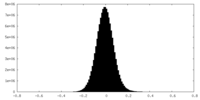
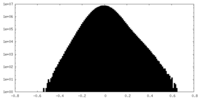
| Density Histograms |
- Sample components
Sample components
+Entire : RyR1 complex with activating ligands in open state
+Supramolecule #1: RyR1 complex with activating ligands in open state
+Supramolecule #2: Ryanodine receptor 1
+Supramolecule #3: Peptidyl-prolyl cis-trans isomerase FKBP1B, Calmodulin-1
+Macromolecule #1: Ryanodine receptor 1
+Macromolecule #2: Peptidyl-prolyl cis-trans isomerase FKBP1B
+Macromolecule #3: Calmodulin-1
+Macromolecule #4: CAFFEINE
+Macromolecule #5: CALCIUM ION
+Macromolecule #6: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #7: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 10 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Model: Quantifoil R2/2 / Mesh: 300 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: TFS Selectris |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Correlation coefficient |
| Output model |  PDB-8dve: |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)