+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Asymmetric Unit of Western Equine Encephalitis Virus | |||||||||
 Map data Map data | Asymmetric unit of western equine encephalitis virus | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Western Equine Encephalitis virus / Virus-Like Particle / VIRUS LIKE PARTICLE | |||||||||
| Function / homology |  Function and homology information Function and homology informationtogavirin / T=4 icosahedral viral capsid / symbiont-mediated suppression of host toll-like receptor signaling pathway / host cell cytoplasm / symbiont-mediated suppression of host gene expression / serine-type endopeptidase activity / fusion of virus membrane with host endosome membrane / symbiont entry into host cell / virion attachment to host cell / host cell nucleus ...togavirin / T=4 icosahedral viral capsid / symbiont-mediated suppression of host toll-like receptor signaling pathway / host cell cytoplasm / symbiont-mediated suppression of host gene expression / serine-type endopeptidase activity / fusion of virus membrane with host endosome membrane / symbiont entry into host cell / virion attachment to host cell / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / proteolysis / RNA binding / membrane Similarity search - Function | |||||||||
| Biological species |  Western equine encephalitis virus Western equine encephalitis virus | |||||||||
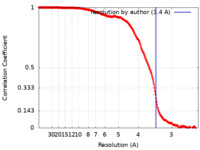
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Pletnev S / Verardi R / Roedeger M / Kwong P | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2023 Journal: Cell / Year: 2023Title: Vaccine elicitation and structural basis for antibody protection against alphaviruses. Authors: Matthew S Sutton / Sergei Pletnev / Victoria Callahan / Sungyoul Ko / Yaroslav Tsybovsky / Tatsiana Bylund / Ryan G Casner / Gabriele Cerutti / Christina L Gardner / Veronica Guirguis / ...Authors: Matthew S Sutton / Sergei Pletnev / Victoria Callahan / Sungyoul Ko / Yaroslav Tsybovsky / Tatsiana Bylund / Ryan G Casner / Gabriele Cerutti / Christina L Gardner / Veronica Guirguis / Raffaello Verardi / Baoshan Zhang / David Ambrozak / Margaret Beddall / Hong Lei / Eun Sung Yang / Tracy Liu / Amy R Henry / Reda Rawi / Arne Schön / Chaim A Schramm / Chen-Hsiang Shen / Wei Shi / Tyler Stephens / Yongping Yang / Maria Burgos Florez / Julie E Ledgerwood / Crystal W Burke / Lawrence Shapiro / Julie M Fox / Peter D Kwong / Mario Roederer /  Abstract: Alphaviruses are RNA viruses that represent emerging public health threats. To identify protective antibodies, we immunized macaques with a mixture of western, eastern, and Venezuelan equine ...Alphaviruses are RNA viruses that represent emerging public health threats. To identify protective antibodies, we immunized macaques with a mixture of western, eastern, and Venezuelan equine encephalitis virus-like particles (VLPs), a regimen that protects against aerosol challenge with all three viruses. Single- and triple-virus-specific antibodies were isolated, and we identified 21 unique binding groups. Cryo-EM structures revealed that broad VLP binding inversely correlated with sequence and conformational variability. One triple-specific antibody, SKT05, bound proximal to the fusion peptide and neutralized all three Env-pseudotyped encephalitic alphaviruses by using different symmetry elements for recognition across VLPs. Neutralization in other assays (e.g., chimeric Sindbis virus) yielded variable results. SKT05 bound backbone atoms of sequence-diverse residues, enabling broad recognition despite sequence variability; accordingly, SKT05 protected mice against Venezuelan equine encephalitis virus, chikungunya virus, and Ross River virus challenges. Thus, a single vaccine-elicited antibody can protect in vivo against a broad range of alphaviruses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27391.map.gz emd_27391.map.gz | 941 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27391-v30.xml emd-27391-v30.xml emd-27391.xml emd-27391.xml | 19.7 KB 19.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27391_fsc.xml emd_27391_fsc.xml | 22.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_27391.png emd_27391.png | 70.8 KB | ||
| Masks |  emd_27391_msk_1.map emd_27391_msk_1.map | 1000 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-27391.cif.gz emd-27391.cif.gz | 6.3 KB | ||
| Others |  emd_27391_half_map_1.map.gz emd_27391_half_map_1.map.gz emd_27391_half_map_2.map.gz emd_27391_half_map_2.map.gz | 926.9 MB 927 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27391 http://ftp.pdbj.org/pub/emdb/structures/EMD-27391 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27391 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27391 | HTTPS FTP |
-Validation report
| Summary document |  emd_27391_validation.pdf.gz emd_27391_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_27391_full_validation.pdf.gz emd_27391_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_27391_validation.xml.gz emd_27391_validation.xml.gz | 30.2 KB | Display | |
| Data in CIF |  emd_27391_validation.cif.gz emd_27391_validation.cif.gz | 39.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27391 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27391 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27391 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27391 | HTTPS FTP |
-Related structure data
| Related structure data |  8deeMC  8decC  8dedC  8defC  8deqC  8derC  8dulC  8dunC  8dwoC  8eeuC  8eevC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27391.map.gz / Format: CCP4 / Size: 1000 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27391.map.gz / Format: CCP4 / Size: 1000 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Asymmetric unit of western equine encephalitis virus | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.245 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_27391_msk_1.map emd_27391_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Asymmetric unit of western equine encephalitis virus
| File | emd_27391_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Asymmetric unit of western equine encephalitis virus | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Asymmetric unit of western equine encephalitis virus
| File | emd_27391_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Asymmetric unit of western equine encephalitis virus | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Western equine encephalitis virus
| Entire | Name:  Western equine encephalitis virus Western equine encephalitis virus |
|---|---|
| Components |
|
-Supramolecule #1: Western equine encephalitis virus
| Supramolecule | Name: Western equine encephalitis virus / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 11039 / Sci species name: Western equine encephalitis virus / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: OTHER / Virus enveloped: Yes / Virus empty: Yes |
|---|
-Macromolecule #1: Spike glycoprotein E1
| Macromolecule | Name: Spike glycoprotein E1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Western equine encephalitis virus Western equine encephalitis virus |
| Molecular weight | Theoretical: 47.36882 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: FEHATTVPNV PGIPYKALVE RAGYAPLNLE ITVVSSELTP STNKEYVTCR FHTVIPSPQV KCCGSLECKA SSKADYTCRV FGGVYPFMW GGAQCFCDSE NTQLSEAYVE FAPDCTIDHA VALKVHTAAL KVGLRIVYGN TTAHLDTFVN GVTPGSSRDL K VIAGPISA ...String: FEHATTVPNV PGIPYKALVE RAGYAPLNLE ITVVSSELTP STNKEYVTCR FHTVIPSPQV KCCGSLECKA SSKADYTCRV FGGVYPFMW GGAQCFCDSE NTQLSEAYVE FAPDCTIDHA VALKVHTAAL KVGLRIVYGN TTAHLDTFVN GVTPGSSRDL K VIAGPISA AFSPFDHKVV IRKGLVYNYD FPEYGAMKPG AFGDIQASSL DATDIVARTD IRLLKPSVKN IHVPYTQAVS GY EMWKNNS GRPLQETAPF GCKIEVEPLR ASNCAYGHIP ISIDIPDAAF VRSSESPTIL EVSCTVADCI YSADFGGSLT LQY KADREG HCPVHSHSTT AVLKEATTHV TAVGSITLHF STSSPQANFI VSLCGKKTTC NAECKPPADH IIGEPHKVDQ EFQA AVSKT SWNWLLALFG GASSLIVVGL IVLVCSSMLI NTRR UniProtKB: Structural polyprotein |
-Macromolecule #2: Spike glycoprotein E2
| Macromolecule | Name: Spike glycoprotein E2 / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Western equine encephalitis virus Western equine encephalitis virus |
| Molecular weight | Theoretical: 46.374848 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: SITDDFTLTS PYLGFCPYCR HSAPCFSPIK IENVWDESDD GSIRIQVSAQ FGYNQAGTAD VTKFRYMSFD HDHDIKEDSM DKIAISTSG PCRRLGHKGY FLLAQCPPGD SVTVSITSGA SENSCTVEKK IRRKFVGREE YLFPPVHGKL VKCHVYDHLK E TSAGYITM ...String: SITDDFTLTS PYLGFCPYCR HSAPCFSPIK IENVWDESDD GSIRIQVSAQ FGYNQAGTAD VTKFRYMSFD HDHDIKEDSM DKIAISTSG PCRRLGHKGY FLLAQCPPGD SVTVSITSGA SENSCTVEKK IRRKFVGREE YLFPPVHGKL VKCHVYDHLK E TSAGYITM HRPGPHAYKS YLEEASGEVY IKPPSGKNVT YECKCGDYST GIVSTRTKMN GCTKAKQCIA YKSDQTKWVF NS PDLIRHT DHSVQGKLHI PFRLTPTVCP VPLAHTPTVT KWFKGITLHL TATRPTLLTT RKLGLRADAT AEWITGTTSR NFS VGREGL EYVWGNHEPV RVWAQESAPG DPHGWPHEII IHYYHRHPVY TVIVLCGVAL AILVGTASSA ACIAKARRDC LTPY ALAPN ATVPTALAVL CCI UniProtKB: Structural polyprotein |
-Macromolecule #3: Capsid protein
| Macromolecule | Name: Capsid protein / type: protein_or_peptide / ID: 3 / Number of copies: 4 / Enantiomer: LEVO / EC number: togavirin |
|---|---|
| Source (natural) | Organism:  Western equine encephalitis virus Western equine encephalitis virus |
| Molecular weight | Theoretical: 29.11473 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MFPYPQLNFP PVYPTNPMAY RDPNPPRCRW RPFRPPLAAQ IEDLRRSIAN LTFKQRSPNP PPGPPPKKKK SAPKPKPTQP KKKKQQAKK TKRKPKPGKR QRMCMKLESD KTFPIMLNGQ VNGYACVVGG RLMKPLHVEG KIDNEQLAAV KLKKASMYDL E YGDVPQNM ...String: MFPYPQLNFP PVYPTNPMAY RDPNPPRCRW RPFRPPLAAQ IEDLRRSIAN LTFKQRSPNP PPGPPPKKKK SAPKPKPTQP KKKKQQAKK TKRKPKPGKR QRMCMKLESD KTFPIMLNGQ VNGYACVVGG RLMKPLHVEG KIDNEQLAAV KLKKASMYDL E YGDVPQNM KSDTLQYTSD KPPGFYNWHH GAVQYENGRF TVPRGVGGKG DSGRPILDNR GRVVAIVLGG ANEGTRTALS VV TWNQKGV TIKDTPEGSE PW UniProtKB: Structural polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 2937 / Average electron dose: 43.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.7000000000000001 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-8dee: |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)