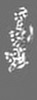+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cardiac thin filament decorated with regulatory M-domain of cardiac myosin binding protein C | |||||||||
 マップデータ マップデータ | cardiac thin filament decorated with THM of regulatory M-domain of cMyBP-C | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | cardiac contraction regulator / muscle protein / MOTOR PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報C zone / regulation of muscle filament sliding / striated muscle myosin thick filament / A band / regulation of striated muscle contraction / cardiac myofibril / Striated Muscle Contraction / regulation of cardiac muscle cell contraction / M band / structural constituent of muscle ...C zone / regulation of muscle filament sliding / striated muscle myosin thick filament / A band / regulation of striated muscle contraction / cardiac myofibril / Striated Muscle Contraction / regulation of cardiac muscle cell contraction / M band / structural constituent of muscle / sarcomere organization / ventricular cardiac muscle tissue morphogenesis / myosin heavy chain binding / myosin binding / ATPase activator activity / heart morphogenesis / cardiac muscle contraction / titin binding / sarcomere / actin binding / cell adhesion / metal ion binding / identical protein binding / cytosol 類似検索 - 分子機能 | |||||||||
| 生物種 |   Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | らせん対称体再構成法 / クライオ電子顕微鏡法 / 解像度: 8.0 Å | |||||||||
 データ登録者 データ登録者 | Risi CM / Galkin VE | |||||||||
| 資金援助 |  米国, 2件 米国, 2件
| |||||||||
 引用 引用 |  ジャーナル: J Mol Biol / 年: 2022 ジャーナル: J Mol Biol / 年: 2022タイトル: Cryo-Electron Microscopy Reveals Cardiac Myosin Binding Protein-C M-Domain Interactions with the Thin Filament. 著者: Cristina M Risi / Edwin Villanueva / Betty Belknap / Rachel L Sadler / Samantha P Harris / Howard D White / Vitold E Galkin /  要旨: Cardiac myosin binding protein C (cMyBP-C) modulates cardiac contraction via direct interactions with cardiac thick (myosin) and thin (actin) filaments (cTFs). While its C-terminal domains (e.g. C8- ...Cardiac myosin binding protein C (cMyBP-C) modulates cardiac contraction via direct interactions with cardiac thick (myosin) and thin (actin) filaments (cTFs). While its C-terminal domains (e.g. C8-C10) anchor cMyBP-C to the backbone of the thick filament, its N-terminal domains (NTDs) (e.g. C0, C1, M, and C2) bind to both myosin and actin to accomplish its dual roles of inhibiting thick filaments and activating cTFs. While the positions of C0, C1 and C2 on cTF have been reported, the binding site of the M-domain on the surface of the cTF is unknown. Here, we used cryo-EM to reveal that the M-domain interacts with actin via helix 3 of its ordered tri-helix bundle region, while the unstructured part of the M-domain does not maintain extensive interactions with actin. We combined the recently obtained structure of the cTF with the positions of all the four NTDs on its surface to propose a complete model of the NTD binding to the cTF. The model predicts that the interactions of the NTDs with the cTF depend on the activation state of the cTF. At the peak of systole, when bound to the extensively activated cTF, NTDs would inhibit actomyosin interactions. In contrast, at falling Ca levels, NTDs would not compete with the myosin heads for binding to the cTF, but would rather promote formation of active cross-bridges at the adjacent regulatory units located at the opposite cTF strand. Our structural data provides a testable model of the cTF regulation by the cMyBP-C. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
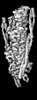
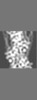
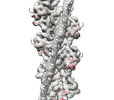
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_25914.map.gz emd_25914.map.gz | 3.7 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-25914-v30.xml emd-25914-v30.xml emd-25914.xml emd-25914.xml | 15.4 KB 15.4 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_25914.png emd_25914.png | 117.1 KB | ||
| Filedesc metadata |  emd-25914.cif.gz emd-25914.cif.gz | 6.3 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25914 http://ftp.pdbj.org/pub/emdb/structures/EMD-25914 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25914 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25914 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_25914_validation.pdf.gz emd_25914_validation.pdf.gz | 386.3 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_25914_full_validation.pdf.gz emd_25914_full_validation.pdf.gz | 385.9 KB | 表示 | |
| XML形式データ |  emd_25914_validation.xml.gz emd_25914_validation.xml.gz | 4.6 KB | 表示 | |
| CIF形式データ |  emd_25914_validation.cif.gz emd_25914_validation.cif.gz | 5.1 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25914 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25914 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25914 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25914 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_25914.map.gz / 形式: CCP4 / 大きさ: 4.2 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_25914.map.gz / 形式: CCP4 / 大きさ: 4.2 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | cardiac thin filament decorated with THM of regulatory M-domain of cMyBP-C | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 これらの図は立方格子座標系で作成されたものです | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.345 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : Complex of cardiac thin filament with C1-M fragment of cardiac my...
| 全体 | 名称: Complex of cardiac thin filament with C1-M fragment of cardiac myosin binding protein C (cMyBP-C) |
|---|---|
| 要素 |
|
-超分子 #1: Complex of cardiac thin filament with C1-M fragment of cardiac my...
| 超分子 | 名称: Complex of cardiac thin filament with C1-M fragment of cardiac myosin binding protein C (cMyBP-C) タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all 詳細: Only triple helix motif of the M-domain is visible in the map. C1 domain is not bound and hence not visible in the map. |
|---|
-分子 #1: cardiac actin
| 分子 | 名称: cardiac actin / タイプ: protein_or_peptide / ID: 1 / コピー数: 6 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 41.830551 KDa |
| 配列 | 文字列: DDEETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IEHGIITNWD DMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSGDGV T HNVPIYEG ...文字列: DDEETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IEHGIITNWD DMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSGDGV T HNVPIYEG YALPHAIMRL DLAGRDLTDY LMKILTERGY SFVTTAEREI VRDIKEKLCY VALDFENEMA TAASSSSLEK SY ELPDGQV ITIGNERFRC PETLFQPSFI GMESAGIHET TYNSIMKCDI DIRKDLYANN VLSGGTTMYP GIADRMQKEI TAL APSTMK IKIIAPPERK YSVWIGGSIL ASLSTFQQMW ISKQEYDEAG PSIVHRKCF UniProtKB: Actin alpha cardiac muscle 1 |
-分子 #2: Myosin-binding protein C, cardiac-type
| 分子 | 名称: Myosin-binding protein C, cardiac-type / タイプ: protein_or_peptide / ID: 2 詳細: only triple helix motif from C1-M construct bound to thin filament is visible in the map and contained in the model コピー数: 6 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 24.803123 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: DDPIGLFVMR PQDGEVTVGG SITFSARVAG ASLLKPPVVK WFKGKWVDLS SKVGQHLQLH DSYDRASKVY LFELHITDAQ PAFTGSYRC EVSTKDKFDC SNFNLTVHEA MGTGDLDLLS AFRRTSLAGG GRRISDSHED TGILDFSSLL KKRDSFRTPR D SKLEAPAE ...文字列: DDPIGLFVMR PQDGEVTVGG SITFSARVAG ASLLKPPVVK WFKGKWVDLS SKVGQHLQLH DSYDRASKVY LFELHITDAQ PAFTGSYRC EVSTKDKFDC SNFNLTVHEA MGTGDLDLLS AFRRTSLAGG GRRISDSHED TGILDFSSLL KKRDSFRTPR D SKLEAPAE EDVWEILRQA PPSEYERIAF QYGVTDLRGM LKRLKGMRRD EKKSTAFQKK LE UniProtKB: Myosin-binding protein C, cardiac-type |
-分子 #3: tropomyosin model
| 分子 | 名称: tropomyosin model / タイプ: protein_or_peptide / ID: 3 / コピー数: 4 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 11.507176 KDa |
| 配列 | 文字列: (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) ...文字列: (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | らせん対称体再構成法 |
| 試料の集合状態 | helical array |
- 試料調製
試料調製
| 緩衝液 | pH: 7 |
|---|---|
| グリッド | モデル: PELCO Ultrathin Carbon with Lacey Carbon / 材質: COPPER / メッシュ: 300 / 前処理 - タイプ: PLASMA CLEANING / 前処理 - 時間: 20 sec. |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 275 K / 装置: FEI VITROBOT MARK IV |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 平均電子線量: 34.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 3.5 µm / 最小 デフォーカス(公称値): 1.0 µm |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 最終 再構成 | 想定した対称性 - らせんパラメータ - Δz: 27.4 Å 想定した対称性 - らせんパラメータ - ΔΦ: -166.7 ° 想定した対称性 - らせんパラメータ - 軸対称性: C1 (非対称) アルゴリズム: BACK PROJECTION / 解像度のタイプ: BY AUTHOR / 解像度: 8.0 Å / 解像度の算出法: FSC 0.143 CUT-OFF / ソフトウェア - 名称: IHRSR / 使用した粒子像数: 14282 |
|---|---|
| 初期モデル | モデルのタイプ: OTHER / 詳細: F-actin model filtered to 100A resolution |
| 最終 角度割当 | タイプ: NOT APPLICABLE / ソフトウェア - 名称: IHRSR |
-原子モデル構築 1
| 初期モデル |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 精密化 | 空間: REAL / プロトコル: FLEXIBLE FIT | ||||||||
| 得られたモデル |  PDB-7tit: |
 ムービー
ムービー コントローラー
コントローラー














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)