[English] 日本語
 Yorodumi
Yorodumi- EMDB-25758: Native human TSH bound to human Thyrotropin receptor in complex w... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Native human TSH bound to human Thyrotropin receptor in complex with miniGs399 (composite structure) | |||||||||
 Map data Map data | Sharpened composite map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | G protein-coupled receptor / Thyroid-stimulating hormone receptor / Thyrotropin receptor / TSH / Thyroid / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationthyroid-stimulating hormone signaling pathway / cellular response to thyrotropin-releasing hormone / thyroid-stimulating hormone receptor activity / cellular response to glycoprotein / positive regulation of steroid biosynthetic process / follicle-stimulating hormone activity / follicle-stimulating hormone complex / pituitary gonadotropin complex / luteinizing hormone secretion / follicle-stimulating hormone secretion ...thyroid-stimulating hormone signaling pathway / cellular response to thyrotropin-releasing hormone / thyroid-stimulating hormone receptor activity / cellular response to glycoprotein / positive regulation of steroid biosynthetic process / follicle-stimulating hormone activity / follicle-stimulating hormone complex / pituitary gonadotropin complex / luteinizing hormone secretion / follicle-stimulating hormone secretion / Thyroxine biosynthesis / Mineralocorticoid biosynthesis / Glycoprotein hormones / Hormone ligand-binding receptors / Reactions specific to the complex N-glycan synthesis pathway / Androgen biosynthesis / regulation of signaling receptor activity / follicle-stimulating hormone signaling pathway / negative regulation of organ growth / thyroid hormone generation / G protein-coupled peptide receptor activity / organ growth / thyroid gland development / PKA activation in glucagon signalling / developmental growth / hair follicle placode formation / anatomical structure morphogenesis / intracellular transport / D1 dopamine receptor binding / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / vascular endothelial cell response to laminar fluid shear stress / renal water homeostasis / activation of adenylate cyclase activity / Hedgehog 'off' state / adenylate cyclase-activating adrenergic receptor signaling pathway / TFAP2 (AP-2) family regulates transcription of growth factors and their receptors / hormone-mediated signaling pathway / regulation of insulin secretion / cellular response to glucagon stimulus / adenylate cyclase activator activity / trans-Golgi network membrane / negative regulation of inflammatory response to antigenic stimulus / hormone activity / bone development / Golgi lumen / platelet aggregation / cognition / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / sensory perception of smell / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / cell-cell signaling / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / signaling receptor activity / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / positive regulation of cold-induced thermogenesis / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / retina development in camera-type eye / G protein activity / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / basolateral plasma membrane / G alpha (s) signalling events Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Faust B / Cheng Y / Manglik A | |||||||||
| Funding support | 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Autoantibody mimicry of hormone action at the thyrotropin receptor. Authors: Bryan Faust / Christian B Billesbølle / Carl-Mikael Suomivuori / Isha Singh / Kaihua Zhang / Nicholas Hoppe / Antonio F M Pinto / Jolene K Diedrich / Yagmur Muftuoglu / Mariusz W ...Authors: Bryan Faust / Christian B Billesbølle / Carl-Mikael Suomivuori / Isha Singh / Kaihua Zhang / Nicholas Hoppe / Antonio F M Pinto / Jolene K Diedrich / Yagmur Muftuoglu / Mariusz W Szkudlinski / Alan Saghatelian / Ron O Dror / Yifan Cheng / Aashish Manglik /  Abstract: Thyroid hormones are vital in metabolism, growth and development. Thyroid hormone synthesis is controlled by thyrotropin (TSH), which acts at the thyrotropin receptor (TSHR). In patients with Graves' ...Thyroid hormones are vital in metabolism, growth and development. Thyroid hormone synthesis is controlled by thyrotropin (TSH), which acts at the thyrotropin receptor (TSHR). In patients with Graves' disease, autoantibodies that activate the TSHR pathologically increase thyroid hormone activity. How autoantibodies mimic thyrotropin function remains unclear. Here we determined cryo-electron microscopy structures of active and inactive TSHR. In inactive TSHR, the extracellular domain lies close to the membrane bilayer. Thyrotropin selects an upright orientation of the extracellular domain owing to steric clashes between a conserved hormone glycan and the membrane bilayer. An activating autoantibody from a patient with Graves' disease selects a similar upright orientation of the extracellular domain. Reorientation of the extracellular domain transduces a conformational change in the seven-transmembrane-segment domain via a conserved hinge domain, a tethered peptide agonist and a phospholipid that binds within the seven-transmembrane-segment domain. Rotation of the TSHR extracellular domain relative to the membrane bilayer is sufficient for receptor activation, revealing a shared mechanism for other glycoprotein hormone receptors that may also extend to other G-protein-coupled receptors with large extracellular domains. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25758.map.gz emd_25758.map.gz | 464.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25758-v30.xml emd-25758-v30.xml emd-25758.xml emd-25758.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25758.png emd_25758.png | 91.5 KB | ||
| Filedesc metadata |  emd-25758.cif.gz emd-25758.cif.gz | 7.3 KB | ||
| Others |  emd_25758_additional_1.map.gz emd_25758_additional_1.map.gz | 245.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25758 http://ftp.pdbj.org/pub/emdb/structures/EMD-25758 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25758 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25758 | HTTPS FTP |
-Related structure data
| Related structure data |  7t9iMC  7t9mC  7t9nC  7utzC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
| EM raw data |  EMPIAR-11120 (Title: Single-particle cryo-electron microscopy final particle stacks and .star files from TSHR complexes EMPIAR-11120 (Title: Single-particle cryo-electron microscopy final particle stacks and .star files from TSHR complexesData size: 828.7 Data #1: Final particle stack for CS-17 Fab-bound TSHR Gs reconstruction [picked particles - single frame - processed] Data #2: Final particle stack for M22 Fab-bound TSHR-Gs reconstruction [picked particles - single frame - processed] Data #3: Final particle stack for TR1402-bound TSHR-Gs reconstruction [picked particles - single frame - processed] Data #4: Final particle stack for native human TSH-bound TSHR-Gs reconstruction [picked particles - single frame - processed]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25758.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25758.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened composite map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.662 Å | ||||||||||||||||||||||||||||||||||||
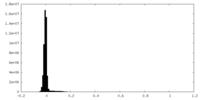
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Unsharpened composite map
| File | emd_25758_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened composite map | ||||||||||||
| Projections & Slices |
| ||||||||||||
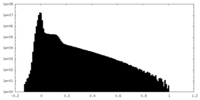
| Density Histograms |
- Sample components
Sample components
-Entire : Native human TSH bound to human Thyrotropin receptor in complex w...
| Entire | Name: Native human TSH bound to human Thyrotropin receptor in complex with miniGs399 (composite structure) |
|---|---|
| Components |
|
-Supramolecule #1: Native human TSH bound to human Thyrotropin receptor in complex w...
| Supramolecule | Name: Native human TSH bound to human Thyrotropin receptor in complex with miniGs399 (composite structure) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#7 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Glycoprotein hormones alpha chain
| Macromolecule | Name: Glycoprotein hormones alpha chain / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 10.217769 KDa |
| Sequence | String: APDVQDCPEC TLQENPFFSQ PGAPILQCMG CCFSRAYPTP LRSKKTMLVQ KNVTSESTCC VAKSYNRVTV MGGFKVENHT ACHCSTCYY HKS UniProtKB: Glycoprotein hormones alpha chain |
-Macromolecule #2: Thyrotropin subunit beta
| Macromolecule | Name: Thyrotropin subunit beta / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.915974 KDa |
| Sequence | String: FCIPTEYTMH IERRECAYCL TINTTICAGY CMTRDINGKL FLPKYALSQD VCTYRDFIYR TVEIPGCPLH VAPYFSYPVA LSCKCGKCN TDYSDCIHEA IKTNYCTKPQ KSY UniProtKB: Thyrotropin subunit beta |
-Macromolecule #3: Nanobody 35
| Macromolecule | Name: Nanobody 35 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 15.398067 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGGG LVQPGGSLRL SCAASGFTFS NYKMNWVRQA PGKGLEWVSD ISQSGASISY TGSVKGRFTI SRDNAKNTLY LQMNSLKPE DTAVYYCARC PAPFTRDCFD VTSTTYAYRG QGTQVTVSSG SEDQVDPRLI DGK |
-Macromolecule #4: Thyrotropin receptor
| Macromolecule | Name: Thyrotropin receptor / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 79.742375 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DYKDDDDGTM GCSSPPCECH QEEDFRVTCK DIQRIPSLPP STQTLKLIET HLRTIPSHAF SNLPNISRIY VSIDVTLQQL ESHSFYNLS KVTHIEIRNT RNLTYIDPDA LKELPLLKFL GIFNTGLKMF PDLTKVYSTD IFFILEITDN PYMTSIPVNA F QGLCNETL ...String: DYKDDDDGTM GCSSPPCECH QEEDFRVTCK DIQRIPSLPP STQTLKLIET HLRTIPSHAF SNLPNISRIY VSIDVTLQQL ESHSFYNLS KVTHIEIRNT RNLTYIDPDA LKELPLLKFL GIFNTGLKMF PDLTKVYSTD IFFILEITDN PYMTSIPVNA F QGLCNETL TLKLYNNGFT SVQGYAFNGT KLDAVYLNKN KYLTVIDKDA FGGVYSGPSL LDVSQTSVTA LPSKGLEHLK EL IARNTWT LKKLPLSLSF LHLTRADLSY PSHCCAFKNQ KKIRGILESL MCNESSMQSL RQRKSVNGQE LKNPQEETLQ AFD SHYDYT ICGDSEDMVC TPKSDEFNPC EDIMGYKFLR IVVWFVSLLA LLGNVFVLLI LLTSHYKLNV PRFLMCNLAF ADFC MGMYL LLIASVDLYT HSEYYNHAID WQTGPGCNTA GFFTVFASEL SVYTLTVITL ERWYAITFAM RLDRKIRLRH ACAIM VGGW VCCFLLALLP LVGISSYAKV SICLPMDTET PLALAYIVFV LTLNIVAFVI VCCCYVKIYI TVRNPQYNPG DKDTKI AKR MAVLIFTDFI CMAPISFYAL SAILNKPLIT VSNSKILLVL FYPLNSCANP FLYAIFTKAF QRDVFILLSK FGICKRQ AQ AYRGQRVPPK NSTDIQVQKV THEMRQGLHN MEDVYELIEN SHLTPKKQGQ ISEEYMQTVL UniProtKB: Thyrotropin receptor |
-Macromolecule #5: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
| Macromolecule | Name: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 30.137025 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GGSLEVLFQG PSGNSKTEDQ RNEEKAQREA NKKIEKQLQK DKQVYRATHR LLLLGADNSG KSTIVKQMRI LHGGSGGSGG TSGIFETKF QVDKVNFHMF DVGGQRDERR KWIQCFNDVT AIIFVVDSSD YNRLQEALNL FKSIWNNRWL RTISVILFLN K QDLLAEKV ...String: GGSLEVLFQG PSGNSKTEDQ RNEEKAQREA NKKIEKQLQK DKQVYRATHR LLLLGADNSG KSTIVKQMRI LHGGSGGSGG TSGIFETKF QVDKVNFHMF DVGGQRDERR KWIQCFNDVT AIIFVVDSSD YNRLQEALNL FKSIWNNRWL RTISVILFLN K QDLLAEKV LAGKSKLEDY FPEFARYTTP EDATPEPGED PRVTRAKYFI RDEFLRISTA SGDGRHYCYP HFTCAVDTEN AR RIFNDCR DIIQRMHLRQ YELL UniProtKB: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short |
-Macromolecule #6: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.786566 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MHHHHHHLEV LFQGPEDQVD PRLIDGKGSS GSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIY AMHWGTDSRL LVSASQDGKL IIWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR E GNVRVSRE ...String: MHHHHHHLEV LFQGPEDQVD PRLIDGKGSS GSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIY AMHWGTDSRL LVSASQDGKL IIWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR E GNVRVSRE LAGHTGYLSC CRFLDDNQIV TSSGDTTCAL WDIETGQQTT TFTGHTGDVM SLSLAPDTRL FVSGACDASA KL WDVREGM CRQTFTGHES DINAICFFPN GNAFATGSDD ATCRLFDLRA DQELMTYSHD NIICGITSVS FSKSGRLLLA GYD DFNCNV WDALKADRAG VLAGHDNRVS CLGVTDDGMA VATGSWDSFL KIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #7: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 7 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #9: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 9 / Number of copies: 6 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 77.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-7t9i: |
 Movie
Movie Controller
Controller







































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)