+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24259 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | State 3 of TcdB and FZD2 at pH5 | |||||||||
 Map data Map data | State 3 of TcdB and FZD2 at pH5 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TOXIN / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationglucosyltransferase activity / host cell cytosol / Transferases; Glycosyltransferases; Hexosyltransferases / cysteine-type peptidase activity / host cell endosome membrane / toxin activity / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / lipid binding / host cell plasma membrane / proteolysis ...glucosyltransferase activity / host cell cytosol / Transferases; Glycosyltransferases; Hexosyltransferases / cysteine-type peptidase activity / host cell endosome membrane / toxin activity / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / lipid binding / host cell plasma membrane / proteolysis / extracellular region / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |  Clostridioides difficile (bacteria) Clostridioides difficile (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.6 Å | |||||||||
 Authors Authors | Jiang M / Zhang J | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Structural Basis for Receptor Recognition of Clostridium difficile Toxin B and its Dissociation upon Acidification Authors: Jiang M / Zhang J | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24259.map.gz emd_24259.map.gz | 255.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24259-v30.xml emd-24259-v30.xml emd-24259.xml emd-24259.xml | 12.5 KB 12.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_24259.png emd_24259.png | 56.9 KB | ||
| Filedesc metadata |  emd-24259.cif.gz emd-24259.cif.gz | 6.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24259 http://ftp.pdbj.org/pub/emdb/structures/EMD-24259 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24259 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24259 | HTTPS FTP |
-Validation report
| Summary document |  emd_24259_validation.pdf.gz emd_24259_validation.pdf.gz | 329.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24259_full_validation.pdf.gz emd_24259_full_validation.pdf.gz | 329.2 KB | Display | |
| Data in XML |  emd_24259_validation.xml.gz emd_24259_validation.xml.gz | 7.4 KB | Display | |
| Data in CIF |  emd_24259_validation.cif.gz emd_24259_validation.cif.gz | 8.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24259 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24259 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24259 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24259 | HTTPS FTP |
-Related structure data
| Related structure data |  7n9qMC  7n8xC  7n95C  7n97C  7n9rC  7n9sC  7n9yC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24259.map.gz / Format: CCP4 / Size: 299.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24259.map.gz / Format: CCP4 / Size: 299.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
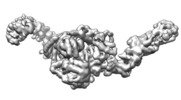
| Annotation | State 3 of TcdB and FZD2 at pH5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06065 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : State 3 of TcdB and FZD2 at pH5
| Entire | Name: State 3 of TcdB and FZD2 at pH5 |
|---|---|
| Components |
|
-Supramolecule #1: State 3 of TcdB and FZD2 at pH5
| Supramolecule | Name: State 3 of TcdB and FZD2 at pH5 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Clostridioides difficile (bacteria) Clostridioides difficile (bacteria) |
| Molecular weight | Theoretical: 250 KDa |
-Macromolecule #1: Toxin B
| Macromolecule | Name: Toxin B / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases |
|---|---|
| Source (natural) | Organism:  Clostridioides difficile (bacteria) Clostridioides difficile (bacteria) |
| Molecular weight | Theoretical: 269.807219 KDa |
| Recombinant expression | Organism:  Bacillus megaterium NBRC 15308 = ATCC 14581 (bacteria) Bacillus megaterium NBRC 15308 = ATCC 14581 (bacteria) |
| Sequence | String: SLVNRKQLEK MANVRFRTQE DEYVAILDAL EEYHNMSENT VVEKYLKLKD INSLTDIYID TYKKSGRNKA LKKFKEYLVT EVLELKNNN LTPVEKNLHF VWIGGQINDT AINYINQWKD VNSDYNVNVF YDSNAFLINT LKKTVVESAI NDTLESFREN L NDPRFDYN ...String: SLVNRKQLEK MANVRFRTQE DEYVAILDAL EEYHNMSENT VVEKYLKLKD INSLTDIYID TYKKSGRNKA LKKFKEYLVT EVLELKNNN LTPVEKNLHF VWIGGQINDT AINYINQWKD VNSDYNVNVF YDSNAFLINT LKKTVVESAI NDTLESFREN L NDPRFDYN KFFRKRMEII YDKQKNFINY YKAQREENPE LIIDDIVKTY LSNEYSKEID ELNTYIEESL NKITQNSGND VR NFEEFKN GESFNLYEQE LVERWNLAAA SDILRISALK EIGGMYLDVD MLPGIQPDLF ESIEKPSSVT VDFWEMTKLE AIM KYKEYI PEYTSEHFDM LDEEVQSSFE SVLASKSDKS EIFSSLGDME ASPLEVKIAF NSKGIINQGL ISVKDSYCSN LIVK QIENR YKILNNSLNP AISEDNDFNT TTNTFIDSIM AEANADNGRF MMELGKYLRV GFFPDVKTTI NLSGPEAYAA AYQDL LMFK EGSMNIHLIE ADLRNFEISK TNISQSTEQE MASLWSFDDA RAKAQFEEYK RNYFEGSLGE DDNLDFSQNI VVDKEY LLE KISSLARSSE RGYIHYIVQL QGDKISYEAA CNLFAKTPYD SVLFQKNIED SEIAYYYNPG DGEIQEIDKY KIPSIIS DR PKIKLTFIGH GKDEFNTDIF AGFDVDSLST EIEAAIDLAK EDISPKSIEI NLLGCNMFSY SINVEETYPG KLLLKVKD K ISELMPSISQ DSIIVSANQY EVRINSEGRR ELLDHSGEWI NKEESIIKDI SSKEYISFNP KENKITVKSK NLPELSTLL QEIRNNSNSS DIELEEKVML TECEINVISN IDTQIVEERI EEAKNLTSDS INYIKDEFKL IESISDALCD LKQQNELEDS HFISFEDIS ETDEGFSIRF INKETGESIF VETEKTIFSE YANHITEEIS KIKGTIFDTV NGKLVKKVNL DTTHEVNTLN A AFFIQSLI EYNSSKESLS NLSVAMKVQV YAQLFSTGLN TITDAAKVVE LVSTALDETI DLLPTLSEGL PIIATIIDGV SL GAAIKEL SETSDPLLRQ EIEAKIGIMA VNLTTATTAI ITSSLGIASG FSILLVPLAG ISAGIPSLVN NELVLRDKAT KVV DYFKHV SLVETEGVFT LLDDKIMMPQ DDLVISEIDF NNNSIVLGKC EIWRMEGGSG HTVTDDIDHF FSAPSITYRE PHLS IYDVL EVQKEELDLS KDLMVLPNAP NRVFAWETGW TPGLRSLEND GTKLLDRIRD NYEGEFYWRY FAFIADALIT TLKPR YEDT NIRINLDSNT RSFIVPIITT EYIREKLSYS FYGSGGTYAL SLSQYNMGIN IELSESDVWI IDVDNVVRDV TIESDK IKK GDLIEGILST LSIEENKIIL NSHEINFSGE VNGSNGFVSL TFSILEGINA IIEVDLLSKS YKLLISGELK ILMLNSN HI QQKIDYIGFN SELQKNIPYS FVDSEGKENG FINGSTKEGL FVSELPDVVL ISKVYMDDSK PSFGYYSNNL KDVKVITK D NVNILTGYYL KDDIKISLSL TLQDEKTIKL NSVHLDESGV AEILKFMNRK GNTNTSDSLM SFLESMNIKS IFVNFLQSN IKFILDANFI ISGTTSIGQF EFICDENDNI QPYFIKFNTL ETNYTLYVGN RQNMIVEPNY DLDDSGDISS TVINFSQKYL YGIDSCVNK VVISPNIYTD EINITPVYET NNTYPEVIVL DANYINEKIN VNINDLSIRY VWSNDGNDFI LMSTSEENKV S QVKIRFVN VFKDKTLANK LSFNFSDKQD VPVSEIILSF TPSYYEDGLI GYDLGLVSLY NEKFYINNFG MMVSGLIYIN DS LYYFKPP VNNLITGFVT VGDDKYYFNP INGGAASIGE TIIDDKNYYF NQSGVLQTGV FSTEDGFKYF APANTLDENL EGE AIDFTG KLIIDENIYY FDDNYRGAVE WKELDGEMHY FSPETGKAFK GLNQIGDYKY YFNSDGVMQK GFVSINDNKH YFDD SGVMK VGYTEIDGKH FYFAENGEMQ IGVFNTEDGF KYFAHHNEDL GNEEGEEISY SGILNFNNKI YYFDDSFTAV VGWKD LEDG SKYYFDEDTA EAYIGLSLIN DGQYYFNDDG IMQVGFVTIN DKVFYFSDSG IIESGVQNID DNYFYIDDNG IVQIGV FDT SDGYKYFAPA NTVNDNIYGQ AVEYSGLVRV GEDVYYFGET YTIETGWIYD MENESDKYYF NPETKKACKG INLIDDI KY YFDEKGIMRT GLISFENNNY YFNENGEMQF GYINIEDKMF YFGEDGVMQI GVFNTPDGFK YFAHQNTLDE NFEGESIN Y TGWLDLDEKR YYFTDEYIAA TGSVIIDGEE YYFDPDTAQL VISE UniProtKB: Toxin B |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















