+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23834 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | T4GALA Engineered Protein Nanocage | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Encapsulin / Nanocage / Nanocompartment / VIRUS LIKE PARTICLE | |||||||||
| Function / homology | Type 1 encapsulin shell protein / Encapsulating protein for peroxidase / : / encapsulin nanocompartment / iron ion transport / intracellular iron ion homeostasis / Type 1 encapsulin shell protein Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.57 Å | |||||||||
 Authors Authors | Andreas MP / Jones JA | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Angew Chem Int Ed Engl / Year: 2021 Journal: Angew Chem Int Ed Engl / Year: 2021Title: Triggered Reversible Disassembly of an Engineered Protein Nanocage*. Authors: Jesse A Jones / Ajitha S Cristie-David / Michael P Andreas / Tobias W Giessen /  Abstract: Protein nanocages play crucial roles in sub-cellular compartmentalization and spatial control in all domains of life and have been used as biomolecular tools for applications in biocatalysis, drug ...Protein nanocages play crucial roles in sub-cellular compartmentalization and spatial control in all domains of life and have been used as biomolecular tools for applications in biocatalysis, drug delivery, and bionanotechnology. The ability to control their assembly state under physiological conditions would further expand their practical utility. To gain such control, we introduced a peptide capable of triggering conformational change at a key structural position in the largest known encapsulin nanocompartment. We report the structure of the resulting engineered nanocage and demonstrate its ability to disassemble and reassemble on demand under physiological conditions. We demonstrate its capacity for in vivo encapsulation of proteins of choice while also demonstrating in vitro cargo loading capabilities. Our results represent a functionally robust addition to the nanocage toolbox and a novel approach for controlling protein nanocage disassembly and reassembly under mild conditions. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23834.map.gz emd_23834.map.gz | 306.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23834-v30.xml emd-23834-v30.xml emd-23834.xml emd-23834.xml | 13.5 KB 13.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23834.png emd_23834.png | 92.2 KB | ||
| Filedesc metadata |  emd-23834.cif.gz emd-23834.cif.gz | 5.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23834 http://ftp.pdbj.org/pub/emdb/structures/EMD-23834 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23834 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23834 | HTTPS FTP |
-Validation report
| Summary document |  emd_23834_validation.pdf.gz emd_23834_validation.pdf.gz | 716.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23834_full_validation.pdf.gz emd_23834_full_validation.pdf.gz | 715.6 KB | Display | |
| Data in XML |  emd_23834_validation.xml.gz emd_23834_validation.xml.gz | 7.7 KB | Display | |
| Data in CIF |  emd_23834_validation.cif.gz emd_23834_validation.cif.gz | 8.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23834 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23834 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23834 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23834 | HTTPS FTP |
-Related structure data
| Related structure data |  7mh2MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23834.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23834.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.40318 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
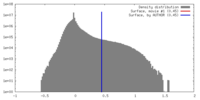
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : T4GALA Engineered Protein Nanocage
| Entire | Name: T4GALA Engineered Protein Nanocage |
|---|---|
| Components |
|
-Supramolecule #1: T4GALA Engineered Protein Nanocage
| Supramolecule | Name: T4GALA Engineered Protein Nanocage / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: T4GALA Engineered Protein Nanocage
| Macromolecule | Name: T4GALA Engineered Protein Nanocage / type: protein_or_peptide / ID: 1 Details: Amino Acids 58-83: synthetic GALA peptide insertion; Amino Acids 305-310: Linker; Amino Acids 311-316: Affinity Tag Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 35.290652 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MNKSQLYPDS PLTDQDFNQL DQTVIEAARR QLVGRRFIEL YGPLGRGMQS VFNDIFMESH EGGGEALAEA LAEALAEALA GGGAKMDFQ GSFDTEVESS RRVNYTIPML YKDFVLYWRD LEQSKALDIP IDFSVAANAA RDVAFLEDQM IFHGSKEFDI P GLMNVKGR ...String: MNKSQLYPDS PLTDQDFNQL DQTVIEAARR QLVGRRFIEL YGPLGRGMQS VFNDIFMESH EGGGEALAEA LAEALAEALA GGGAKMDFQ GSFDTEVESS RRVNYTIPML YKDFVLYWRD LEQSKALDIP IDFSVAANAA RDVAFLEDQM IFHGSKEFDI P GLMNVKGR LTHLIGNWYE SGNAFQDIVE ARNKLLEMNH NGPYALVLSP ELYSLLHRVH KDTNVLEIEH VRELITAGVF QS PVLKGKS GVIVNTGRNN LDLAISEDFE TAYLGEEGMN HPFRVYETVV LRIKRPAAIC TLIDPEEGGG GGGHHHHHH UniProtKB: Type 1 encapsulin shell protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.76 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Details: 60 seconds, 5 mA | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV Details: Blot force: 20 Blot time: 4 seconds Wait time: 0 seconds. |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 1259 / Average exposure time: 8.0 sec. / Average electron dose: 62.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -1.8 µm / Nominal defocus min: -1.3 µm / Nominal magnification: 45000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Applied symmetry - Point group: I (icosahedral) / Resolution.type: BY AUTHOR / Resolution: 3.57 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 2.15.00) / Software - details: Homogenous Refinement / Number images used: 6707 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION / Software - Name: cryoSPARC (ver. 2.15.00) |
| Final angle assignment | Type: ANGULAR RECONSTITUTION / Software - Name: cryoSPARC (ver. 2.15.00) |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Details | Model was initially docked in Chimera using Fit to Map command. Chains were manually refined in Coot with rigid body refinement, chain refinement, and iterative real space refinements. ASU was then refined using phenix.real_space_refine with default parameters. NCS operators were applied and refined again with NCS contstraints, global minimization, and ADP refinement. |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 81.22 / Target criteria: Correlation Coefficient |
| Output model |  PDB-7mh2: |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)