+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23827 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of C9orf72:SMCR8:WDR41 in complex with ARF1 | |||||||||
 Map data Map data | postprocess with deepemhancer "tightTarget" model | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | complex / autophagy / ALS / GAP / small GTPase / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationmitotic cleavage furrow ingression / trans-Golgi Network Vesicle Budding / Atg1/ULK1 kinase complex / Glycosphingolipid transport / regulation of receptor internalization / late endosome to lysosome transport / Intra-Golgi traffic / regulation of Arp2/3 complex-mediated actin nucleation / negative regulation of immune response / regulation of TORC1 signaling ...mitotic cleavage furrow ingression / trans-Golgi Network Vesicle Budding / Atg1/ULK1 kinase complex / Glycosphingolipid transport / regulation of receptor internalization / late endosome to lysosome transport / Intra-Golgi traffic / regulation of Arp2/3 complex-mediated actin nucleation / negative regulation of immune response / regulation of TORC1 signaling / autophagosome-lysosome fusion / Synthesis of PIPs at the Golgi membrane / regulation of actin filament organization / guanyl-nucleotide exchange factor complex / negative regulation of autophagosome assembly / regulation of autophagosome assembly / Nef Mediated CD4 Down-regulation / dendritic spine organization / Flemming body / regulation of synaptic vesicle cycle / axon extension / positive regulation of autophagosome maturation / long-term synaptic depression / negative regulation of exocytosis / COPI-dependent Golgi-to-ER retrograde traffic / Lysosome Vesicle Biogenesis / negative regulation of macroautophagy / Golgi Associated Vesicle Biogenesis / negative regulation of protein phosphorylation / Synthesis of PIPs at the plasma membrane / protein kinase inhibitor activity / cell leading edge / positive regulation of macroautophagy / main axon / intracellular copper ion homeostasis / positive regulation of TOR signaling / axonal growth cone / COPI-mediated anterograde transport / presynaptic cytosol / vesicle-mediated transport / stress granule assembly / MHC class II antigen presentation / Gene and protein expression by JAK-STAT signaling after Interleukin-12 stimulation / GTPase activator activity / autophagosome / guanyl-nucleotide exchange factor activity / hippocampal mossy fiber to CA3 synapse / sarcomere / small monomeric GTPase / cell projection / intracellular protein transport / P-body / cellular response to virus / small GTPase binding / autophagy / endocytosis / cytoplasmic stress granule / presynapse / regulation of protein localization / nuclear membrane / perikaryon / lysosome / postsynapse / endosome / neuron projection / postsynaptic density / regulation of autophagy / Golgi membrane / protein domain specific binding / negative regulation of gene expression / lysosomal membrane / focal adhesion / intracellular membrane-bounded organelle / GTPase activity / dendrite / protein kinase binding / chromatin / GTP binding / glutamatergic synapse / magnesium ion binding / protein-containing complex / extracellular space / RNA binding / extracellular exosome / nucleoplasm / nucleus / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.94 Å | |||||||||
 Authors Authors | Su M-Y / Hurley JH | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Structural basis for the ARF GAP activity and specificity of the C9orf72 complex. Authors: Ming-Yuan Su / Simon A Fromm / Jonathan Remis / Daniel B Toso / James H Hurley /    Abstract: Mutation of C9ORF72 is the most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontal temporal degeneration (FTD), which is attributed to both a gain and loss of function. C9orf72 ...Mutation of C9ORF72 is the most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontal temporal degeneration (FTD), which is attributed to both a gain and loss of function. C9orf72 forms a complex with SMCR8 and WDR41, which was reported to have GTPase activating protein activity toward ARF proteins, RAB8A, and RAB11A. We determined the cryo-EM structure of ARF1-GDP-BeF bound to C9orf72:SMCR8:WDR41. The SMCR8 and C9orf72 domains form the binding pocket for ARF1. One face of the C9orf72 domain holds ARF1 in place, while the SMCR8 positions the catalytic finger Arg147 in the ARF1 active site. Mutations in interfacial residues of ARF1 and C9orf72 reduced or eliminated GAP activity. RAB8A GAP required ~10-fold higher concentrations of the C9orf72 complex than for ARF1. These data support a specific function for the C9orf72 complex as an ARF GAP. The structure also provides a model for the active forms of the longin domain GAPs of FLCN and NPRL2 that regulate the Rag GTPases of the mTORC1 pathway. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23827.map.gz emd_23827.map.gz | 62.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23827-v30.xml emd-23827-v30.xml emd-23827.xml emd-23827.xml | 18.5 KB 18.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23827.png emd_23827.png | 37.9 KB | ||
| Filedesc metadata |  emd-23827.cif.gz emd-23827.cif.gz | 7.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23827 http://ftp.pdbj.org/pub/emdb/structures/EMD-23827 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23827 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23827 | HTTPS FTP |
-Validation report
| Summary document |  emd_23827_validation.pdf.gz emd_23827_validation.pdf.gz | 353.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23827_full_validation.pdf.gz emd_23827_full_validation.pdf.gz | 353.1 KB | Display | |
| Data in XML |  emd_23827_validation.xml.gz emd_23827_validation.xml.gz | 6.2 KB | Display | |
| Data in CIF |  emd_23827_validation.cif.gz emd_23827_validation.cif.gz | 7.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23827 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23827 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23827 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23827 | HTTPS FTP |
-Related structure data
| Related structure data |  7mgeMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23827.map.gz / Format: CCP4 / Size: 70.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23827.map.gz / Format: CCP4 / Size: 70.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | postprocess with deepemhancer "tightTarget" model | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.94 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
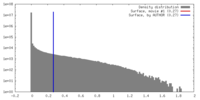
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Complex of C9orf72:SMCR8:WDR41 with ARF1
| Entire | Name: Complex of C9orf72:SMCR8:WDR41 with ARF1 |
|---|---|
| Components |
|
-Supramolecule #1: Complex of C9orf72:SMCR8:WDR41 with ARF1
| Supramolecule | Name: Complex of C9orf72:SMCR8:WDR41 with ARF1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: WD repeat-containing protein 41
| Macromolecule | Name: WD repeat-containing protein 41 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 51.783805 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MLRWLIGGGR EPQGLAEKSP LQTIGEEQTQ NPYTELLVLK AHHDIVRFLV QLDDYRFASA GDDGIVVVWN AQTGEKLLEL NGHTQKITA IITFPSLESC EEKNQLILTA SADRTVIVWD GDTTRQVQRI SCFQSTVKCL TVLQRLDVWL SGGNDLCVWN R KLDLLCKT ...String: MLRWLIGGGR EPQGLAEKSP LQTIGEEQTQ NPYTELLVLK AHHDIVRFLV QLDDYRFASA GDDGIVVVWN AQTGEKLLEL NGHTQKITA IITFPSLESC EEKNQLILTA SADRTVIVWD GDTTRQVQRI SCFQSTVKCL TVLQRLDVWL SGGNDLCVWN R KLDLLCKT SHLSDTGISA LVEIPKNCVV AAVGKELIIF RLVAPTEGSL EWDILEVKRL LDHQDNILSL INVNDLSFVT GS HVGELII WDALDWTMQA YERNFWDPSP QLDTQQEIKL CQKSNDISIH HFTCDEENVF AAVGRGLYVY SLQMKRVIAC QKT AHDSNV LHVARLPNRQ LISCSEDGSV RIWELREKQQ LAAEPVPTGF FNMWGFGRVS KQASQPVKKQ QENATSCSLE LIGD LIGHS SSVEMFLYFE DHGLVTCSAD HLIILWKNGE RESGLRSLRL FQKLEENGDL YLAV UniProtKB: WD repeat-containing protein 41 |
-Macromolecule #2: Guanine nucleotide exchange protein SMCR8
| Macromolecule | Name: Guanine nucleotide exchange protein SMCR8 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 105.149094 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MISAPDVVAF TKEEEYEEEP YNEPALPEEY SVPLFPFASQ GANPWSKLSG AKFSRDFILI SEFSEQVGPQ PLLTIPNDTK VFGTFDLNY FSLRIMSVDY QASFVGHPPG SAYPKLNFVE DSKVVLGDSK EGAFAYVHHL TLYDLEARGF VRPFCMAYIS A DQHKIMQQ ...String: MISAPDVVAF TKEEEYEEEP YNEPALPEEY SVPLFPFASQ GANPWSKLSG AKFSRDFILI SEFSEQVGPQ PLLTIPNDTK VFGTFDLNY FSLRIMSVDY QASFVGHPPG SAYPKLNFVE DSKVVLGDSK EGAFAYVHHL TLYDLEARGF VRPFCMAYIS A DQHKIMQQ FQELSAEFSR ASECLKTGNR KAFAGELEKK LKDLDYTRTV LHTETEIQKK ANDKGFYSSQ AIEKANELAS VE KSIIEHQ DLLKQIRSYP HRKLKGHDLC PGEMEHIQDQ ASQASTTSNP DESADTDLYT CRPAYTPKLI KAKSTKCFDK KLK TLEELC DTEYFTQTLA QLSHIEHMFR GDLCYLLTSQ IDRALLKQQH ITNFLFEDFV EVDDRMVEKQ ESIPSKPSQD RPPS SSLEE CPIPKVLISV GSYKSSVESV LIKMEQELGD EEYKEVEVTE LSSFDPQENL DYLDMDMKGS ISSGESIEVL GTEKS TSVL SKSDSQASLT VPLSPQVVRS KAVSHRTISE DSIEVLSTCP SEALIPDDFK ASYPSAINEE ESYPDGNEGA IRFQAS ISP PELGETEEGS IENTPSQIDS SCCIGKESDG QLVLPSTPAH THSDEDGVVS SPPQRHRQKD QGFRVDFSVE NANPSSR DN SCEGFPAYEL DPSHLLASRD ISKTSLDNYS DTTSYVSSVA STSSDRIPSA YPAGLSSDRH KKRAGQNALK FIRQYPFA H PAIYSLLSGR TLVVLGEDEA IVRKLVTALA IFVPSYGCYA KPVKHWASSP LHIMDFQKWK LIGLQRVASP AGAGTLHAL SRYSRYTSIL DLDNKTLRCP LYRGTLVPRL ADHRTQIKRG STYYLHVQSM LTQLCSKAFL YTFCHHLHLP THDKETEELV ASRQMSFLK LTLGLVNEDV RVVQYLAELL KLHYMQESPG TSHPMLRFDY VPSFLYKI UniProtKB: Guanine nucleotide exchange protein SMCR8 |
-Macromolecule #3: Guanine nucleotide exchange C9orf72
| Macromolecule | Name: Guanine nucleotide exchange C9orf72 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 54.391477 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSTLCPPPSP AVAKTEIALS GKSPLLAATF AYWDNILGPR VRHIWAPKTE QVLLSDGEIT FLANHTLNGE ILRNAESGAI DVKFFVLSE KGVIIVSLIF DGNWNGDRST YGLSIILPQT ELSFYLPLHR VCVDRLTHII RKGRIWMHKE RQENVQKIIL E GTERMEDQ ...String: MSTLCPPPSP AVAKTEIALS GKSPLLAATF AYWDNILGPR VRHIWAPKTE QVLLSDGEIT FLANHTLNGE ILRNAESGAI DVKFFVLSE KGVIIVSLIF DGNWNGDRST YGLSIILPQT ELSFYLPLHR VCVDRLTHII RKGRIWMHKE RQENVQKIIL E GTERMEDQ GQSIIPMLTG EVIPVMELLS SMKSHSVPEE IDIADTVLND DDIGDSCHEG FLLNAISSHL QTCGCSVVVG SS AEKVNKI VRTLCLFLTP AERKCSRLCE AESSFKYESG LFVQGLLKDS TGSFVLPFRQ VMYAPYPTTH IDVDVNTVKQ MPP CHEHIY NQRRYMRSEL TAFWRATSEE DMAQDTIIYT DESFTPDLNI FQDVLHRDTL VKAFLDQVFQ LKPGLSLRST FLAQ FLLVL HRKALTLIKY IEDDTQKGKK PFKSLRNLKI DLDLTAEGDL NIIMALAEKI KPGLHSFIFG RPFYTSVQER DVLMT F UniProtKB: Guanine nucleotide exchange factor C9orf72 |
-Macromolecule #4: ADP-ribosylation factor 1
| Macromolecule | Name: ADP-ribosylation factor 1 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 18.994572 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: ATEMRILMVG LDAAGKTTIL YKLKLGEIVT TIPTIGFNVE TVEYKNISFT VWDVGGQDKI RPLWRHYFQN TQGLIFVVDS NDRERVNEA REELMRMLAE DELRDAVLLV FANKQDLPNA MNAAEITDKL GLHSLRHRNW YIQATCATSG DGLYEGLDWL S NQLRNQ UniProtKB: ADP-ribosylation factor 1 |
-Macromolecule #5: GUANOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-DIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 1 / Formula: GDP |
|---|---|
| Molecular weight | Theoretical: 443.201 Da |
| Chemical component information |  ChemComp-GDP: |
-Macromolecule #6: BERYLLIUM TRIFLUORIDE ION
| Macromolecule | Name: BERYLLIUM TRIFLUORIDE ION / type: ligand / ID: 6 / Number of copies: 1 / Formula: BEF |
|---|---|
| Molecular weight | Theoretical: 66.007 Da |
| Chemical component information |  ChemComp-BEF: |
-Macromolecule #7: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 7 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.16 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Model: C-flat-1.2/1.3 / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
| Details | concentration range of 0.16-0.2 mg/ml |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-7mge: |
 Movie
Movie Controller
Controller



































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)