+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23786 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of SARS-CoV-2 NSP15 NendoU at pH 6.0 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | nsp15 / sars-cov-2 / nendoU / coronavirus / covid-19 / VIRAL PROTEIN / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationprotein guanylyltransferase activity / RNA endonuclease activity producing 3'-phosphomonoesters, hydrolytic mechanism / 5'-3' RNA helicase activity / Lyases; Phosphorus-oxygen lyases / mRNA guanylyltransferase activity / Assembly of the SARS-CoV-2 Replication-Transcription Complex (RTC) / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of TBK1 activity / Maturation of replicase proteins / TRAF3-dependent IRF activation pathway / ISG15-specific peptidase activity ...protein guanylyltransferase activity / RNA endonuclease activity producing 3'-phosphomonoesters, hydrolytic mechanism / 5'-3' RNA helicase activity / Lyases; Phosphorus-oxygen lyases / mRNA guanylyltransferase activity / Assembly of the SARS-CoV-2 Replication-Transcription Complex (RTC) / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of TBK1 activity / Maturation of replicase proteins / TRAF3-dependent IRF activation pathway / ISG15-specific peptidase activity / Transcription of SARS-CoV-2 sgRNAs / snRNP Assembly / Translation of Replicase and Assembly of the Replication Transcription Complex / Replication of the SARS-CoV-2 genome / Hydrolases; Acting on ester bonds; Exoribonucleases producing 5'-phosphomonoesters / double membrane vesicle viral factory outer membrane / SARS coronavirus main proteinase / host cell endoplasmic reticulum-Golgi intermediate compartment / host cell endosome / 3'-5'-RNA exonuclease activity / 5'-3' DNA helicase activity / symbiont-mediated degradation of host mRNA / mRNA guanylyltransferase / symbiont-mediated suppression of host ISG15-protein conjugation / symbiont-mediated suppression of host toll-like receptor signaling pathway / G-quadruplex RNA binding / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of IRF3 activity / omega peptidase activity / mRNA (guanine-N7)-methyltransferase / methyltransferase cap1 / SARS-CoV-2 modulates host translation machinery / host cell Golgi apparatus / symbiont-mediated suppression of host NF-kappaB cascade / DNA helicase / symbiont-mediated perturbation of host ubiquitin-like protein modification / methyltransferase cap1 activity / ubiquitinyl hydrolase 1 / mRNA 5'-cap (guanine-N7-)-methyltransferase activity / cysteine-type deubiquitinase activity / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / lyase activity / single-stranded RNA binding / viral protein processing / host cell perinuclear region of cytoplasm / host cell endoplasmic reticulum membrane / RNA helicase / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / copper ion binding / symbiont-mediated suppression of host gene expression / viral translational frameshifting / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / lipid binding / host cell nucleus / SARS-CoV-2 activates/modulates innate and adaptive immune responses / ATP hydrolysis activity / proteolysis / RNA binding / zinc ion binding / ATP binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
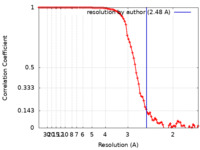
| Method | single particle reconstruction / cryo EM / Resolution: 2.48 Å | |||||||||
 Authors Authors | Godoy AS / Song Y | |||||||||
| Funding support |  Brazil, 1 items Brazil, 1 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2023 Journal: Nucleic Acids Res / Year: 2023Title: Allosteric regulation and crystallographic fragment screening of SARS-CoV-2 NSP15 endoribonuclease. Authors: Andre Schutzer Godoy / Aline Minalli Nakamura / Alice Douangamath / Yun Song / Gabriela Dias Noske / Victor Oliveira Gawriljuk / Rafaela Sachetto Fernandes / Humberto D Muniz Pereira / ...Authors: Andre Schutzer Godoy / Aline Minalli Nakamura / Alice Douangamath / Yun Song / Gabriela Dias Noske / Victor Oliveira Gawriljuk / Rafaela Sachetto Fernandes / Humberto D Muniz Pereira / Ketllyn Irene Zagato Oliveira / Daren Fearon / Alexandre Dias / Tobias Krojer / Michael Fairhead / Alisa Powell / Louise Dunnet / Jose Brandao-Neto / Rachael Skyner / Rod Chalk / Dávid Bajusz / Miklós Bege / Anikó Borbás / György Miklós Keserű / Frank von Delft / Glaucius Oliva /      Abstract: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19). The NSP15 endoribonuclease enzyme, known as NendoU, is highly conserved and ...Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19). The NSP15 endoribonuclease enzyme, known as NendoU, is highly conserved and plays a critical role in the ability of the virus to evade the immune system. NendoU is a promising target for the development of new antiviral drugs. However, the complexity of the enzyme's structure and kinetics, along with the broad range of recognition sequences and lack of structural complexes, hampers the development of inhibitors. Here, we performed enzymatic characterization of NendoU in its monomeric and hexameric form, showing that hexamers are allosteric enzymes with a positive cooperative index, and with no influence of manganese on enzymatic activity. Through combining cryo-electron microscopy at different pHs, X-ray crystallography and biochemical and structural analysis, we showed that NendoU can shift between open and closed forms, which probably correspond to active and inactive states, respectively. We also explored the possibility of NendoU assembling into larger supramolecular structures and proposed a mechanism for allosteric regulation. In addition, we conducted a large fragment screening campaign against NendoU and identified several new allosteric sites that could be targeted for the development of new inhibitors. Overall, our findings provide insights into the complex structure and function of NendoU and offer new opportunities for the development of inhibitors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23786.map.gz emd_23786.map.gz | 58.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23786-v30.xml emd-23786-v30.xml emd-23786.xml emd-23786.xml | 18.3 KB 18.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23786_fsc.xml emd_23786_fsc.xml | 8.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_23786.png emd_23786.png | 32.2 KB | ||
| Masks |  emd_23786_msk_1.map emd_23786_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-23786.cif.gz emd-23786.cif.gz | 5.8 KB | ||
| Others |  emd_23786_additional_1.map.gz emd_23786_additional_1.map.gz emd_23786_half_map_1.map.gz emd_23786_half_map_1.map.gz emd_23786_half_map_2.map.gz emd_23786_half_map_2.map.gz | 31.8 MB 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23786 http://ftp.pdbj.org/pub/emdb/structures/EMD-23786 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23786 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23786 | HTTPS FTP |
-Related structure data
| Related structure data |  7me0MC  5s6xC  5s6yC  5s6zC  5s70C  5s71C  5s72C  5sa4C  5sa5C  5sa6C  5sa7C  5sa8C  5sa9C  5saaC  5sabC  5sacC  5sadC  5saeC  5safC  5sagC  5sahC  5saiC  5sbfC  7kegC  7kehC  7kf4C  7n7rC  7n7uC  7n7wC  7n7yC  7n83C  7rb0C  7rb2C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10794 (Title: Cryo-EM structure of SARS-CoV-2 NSP15 NendoU at pH 6.0 EMPIAR-10794 (Title: Cryo-EM structure of SARS-CoV-2 NSP15 NendoU at pH 6.0Data size: 359.3 Data #1: Aligned micrographs of SARS-CoV-2 NSP15 NendoU at pH 6.0 [micrographs - single frame]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23786.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23786.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.831 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
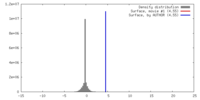
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_23786_msk_1.map emd_23786_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
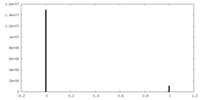
| Density Histograms |
-Additional map: #1
| File | emd_23786_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
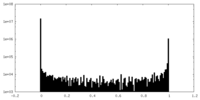
| Density Histograms |
-Half map: #1
| File | emd_23786_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
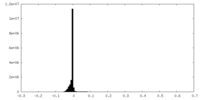
| Density Histograms |
-Half map: #2
| File | emd_23786_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Structure of SARS-CoV-2 NSP15
| Entire | Name: Structure of SARS-CoV-2 NSP15 |
|---|---|
| Components |
|
-Supramolecule #1: Structure of SARS-CoV-2 NSP15
| Supramolecule | Name: Structure of SARS-CoV-2 NSP15 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Uridylate-specific endoribonuclease
| Macromolecule | Name: Uridylate-specific endoribonuclease / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO / EC number: Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 42.117844 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKHHHHHHPM SDYDIPTTEN LYFQGAMSLE NVAFNVVNKG HFDGQQGEVP VSIINNTVYT KVDGVDVELF ENKTTLPVNV AFELWAKRN IKPVPEVKIL NNLGVDIAAN TVIWDYKRDA PAHISTIGVC SMTDIAKKPT ETICAPLTVF FDGRVDGQVD L FRNARNGV ...String: MKHHHHHHPM SDYDIPTTEN LYFQGAMSLE NVAFNVVNKG HFDGQQGEVP VSIINNTVYT KVDGVDVELF ENKTTLPVNV AFELWAKRN IKPVPEVKIL NNLGVDIAAN TVIWDYKRDA PAHISTIGVC SMTDIAKKPT ETICAPLTVF FDGRVDGQVD L FRNARNGV LITEGSVKGL QPSVGPKQAS LNGVTLIGEA VKTQFNYYKK VDGVVQQLPE TYFTQSRNLQ EFKPRSQMEI DF LELAMDE FIERYKLEGY AFEHIVYGDF SHSQLGGLHL LIGLAKRFKE SPFELEDFIP MDSTVKNYFI TDAQTGSSKC VCS VIDLLL DDFVEIIKSQ DLSVVSKVVK VTIDYTEISF MLWCKDGHVE TFYPKLQ UniProtKB: Replicase polyprotein 1ab |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 6 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 41.70522228 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

























 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)