+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23460 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
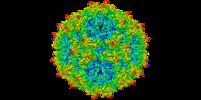
| Title | Gorilla Bocavirus 1 Capsid | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Icosahedral Capsid / Gorilla Bocavirus / GBoV / VIRUS / Parvovirus | |||||||||
| Function / homology | Parvovirus coat protein VP2 / Parvovirus coat protein VP1/VP2 / Parvovirus coat protein VP1/VP2 / Capsid/spike protein, ssDNA virus / T=1 icosahedral viral capsid / structural molecule activity / Capsid protein Function and homology information Function and homology information | |||||||||
| Biological species |  Bocavirus gorilla/GBoV1/2009 / Bocavirus gorilla/GBoV1/2009 /  Gorilla bocavirus Gorilla bocavirus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.76 Å | |||||||||
 Authors Authors | Yu JC / Mietzsch M | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Viruses / Year: 2021 Journal: Viruses / Year: 2021Title: Characterization of the GBoV1 Capsid and Its Antibody Interactions. Authors: Jennifer Chun Yu / Mario Mietzsch / Amriti Singh / Alberto Jimenez Ybargollin / Shweta Kailasan / Paul Chipman / Nilakshee Bhattacharya / Julia Fakhiri / Dirk Grimm / Amit Kapoor / Indrė ...Authors: Jennifer Chun Yu / Mario Mietzsch / Amriti Singh / Alberto Jimenez Ybargollin / Shweta Kailasan / Paul Chipman / Nilakshee Bhattacharya / Julia Fakhiri / Dirk Grimm / Amit Kapoor / Indrė Kučinskaitė-Kodzė / Aurelija Žvirblienė / Maria Söderlund-Venermo / Robert McKenna / Mavis Agbandje-McKenna /    Abstract: Human bocavirus 1 (HBoV1) has gained attention as a gene delivery vector with its ability to infect polarized human airway epithelia and 5.5 kb genome packaging capacity. Gorilla bocavirus 1 (GBoV1) ...Human bocavirus 1 (HBoV1) has gained attention as a gene delivery vector with its ability to infect polarized human airway epithelia and 5.5 kb genome packaging capacity. Gorilla bocavirus 1 (GBoV1) VP3 shares 86% amino acid sequence identity with HBoV1 but has better transduction efficiency in several human cell types. Here, we report the capsid structure of GBoV1 determined to 2.76 Å resolution using cryo-electron microscopy (cryo-EM) and its interaction with mouse monoclonal antibodies (mAbs) and human sera. GBoV1 shares capsid surface morphologies with other parvoviruses, with a channel at the 5-fold symmetry axis, protrusions surrounding the 3-fold axis and a depression at the 2-fold axis. A 2/5-fold wall separates the 2-fold and 5-fold axes. Compared to HBoV1, differences are localized to the 3-fold protrusions. Consistently, native dot immunoblots and cryo-EM showed cross-reactivity and binding, respectively, by a 5-fold targeted HBoV1 mAb, 15C6. Surprisingly, recognition was observed for one out of three 3-fold targeted mAbs, 12C1, indicating some structural similarity at this region. In addition, GBoV1, tested against 40 human sera, showed the similar rates of seropositivity as HBoV1. Immunogenic reactivity against parvoviral vectors is a significant barrier to efficient gene delivery. This study is a step towards optimizing bocaparvovirus vectors with antibody escape properties. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23460.map.gz emd_23460.map.gz | 227 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23460-v30.xml emd-23460-v30.xml emd-23460.xml emd-23460.xml | 12.3 KB 12.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23460.png emd_23460.png | 320 KB | ||
| Filedesc metadata |  emd-23460.cif.gz emd-23460.cif.gz | 5.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23460 http://ftp.pdbj.org/pub/emdb/structures/EMD-23460 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23460 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23460 | HTTPS FTP |
-Validation report
| Summary document |  emd_23460_validation.pdf.gz emd_23460_validation.pdf.gz | 640 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23460_full_validation.pdf.gz emd_23460_full_validation.pdf.gz | 639.5 KB | Display | |
| Data in XML |  emd_23460_validation.xml.gz emd_23460_validation.xml.gz | 7.5 KB | Display | |
| Data in CIF |  emd_23460_validation.cif.gz emd_23460_validation.cif.gz | 8.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23460 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23460 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23460 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23460 | HTTPS FTP |
-Related structure data
| Related structure data |  7lnkMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23460.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23460.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.072 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Gorilla bocavirus
| Entire | Name:  Gorilla bocavirus Gorilla bocavirus |
|---|---|
| Components |
|
-Supramolecule #1: Gorilla bocavirus
| Supramolecule | Name: Gorilla bocavirus / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 1511879 / Sci species name: Gorilla bocavirus / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: No |
|---|
-Macromolecule #1: Capsid protein
| Macromolecule | Name: Capsid protein / type: protein_or_peptide / ID: 1 / Number of copies: 60 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Bocavirus gorilla/GBoV1/2009 Bocavirus gorilla/GBoV1/2009 |
| Molecular weight | Theoretical: 57.548246 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSGVGISTGG WVGGSHFADK YVITKNTRQF ITSIQNGHLY KTEIINPSNG NGKSQRCVTT PWTYFNFNQY SCHFSPQDWQ RLTNEYKRF RPKAMLVKIY NLQIKQILSN GADTTYNNDL TAGVHIFCDG EHAYPNASHP WDEDVMPDLP YKTWKLFQYG Y IPILNELA ...String: GSGVGISTGG WVGGSHFADK YVITKNTRQF ITSIQNGHLY KTEIINPSNG NGKSQRCVTT PWTYFNFNQY SCHFSPQDWQ RLTNEYKRF RPKAMLVKIY NLQIKQILSN GADTTYNNDL TAGVHIFCDG EHAYPNASHP WDEDVMPDLP YKTWKLFQYG Y IPILNELA DLDGTTAGGT ATEKAILYQM PFFMLENSDH EVLRTGESSE FTFNFDCEWV NNERAYIPPG LMFNPKVPTR RV QYIRQNG QTTASTSRIE PYSKPTSWMT GPGFLGGQRV GPATSDTAPY MVCTKPDGVY INTGAAGYGS GFDPPSGSLA PTD LEYKLQ WYQTPEDTGN NGNIIANPSL SMLRDQLLYR GNQTTYNLNA DVWMFPNQIW DRYPITREHP IWCKKPRADK NTII DPFDG SIAMDHPPGT IFIKMAKIPV PSSTNADSYL NIYCTGQVSC EIVWEVERYV TKNWRPERRH TALGMSIGGT ENVSP TYHV DSAGTYIQPT TFDQCMPVKT NINKVL UniProtKB: Capsid protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) X (Row.)
X (Row.) Y (Col.)
Y (Col.)





















