[English] 日本語
 Yorodumi
Yorodumi- EMDB-23450: Cryo-EM structure of human p97 in complex with Npl4/Ufd1 and poly... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23450 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human p97 in complex with Npl4/Ufd1 and polyubiquitinated Ub-Eos (CHAPSO, Class 2, Open State) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | p97 / NMS-873 / single-particle cryo-EM / translocation / allosteric inhibition / TRANSLOCASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationcytoplasmic ubiquitin ligase complex / flavin adenine dinucleotide catabolic process / VCP-NSFL1C complex / endoplasmic reticulum stress-induced pre-emptive quality control / endosome to lysosome transport via multivesicular body sorting pathway / BAT3 complex binding / cellular response to arsenite ion / protein-DNA covalent cross-linking repair / Derlin-1 retrotranslocation complex / positive regulation of protein K63-linked deubiquitination ...cytoplasmic ubiquitin ligase complex / flavin adenine dinucleotide catabolic process / VCP-NSFL1C complex / endoplasmic reticulum stress-induced pre-emptive quality control / endosome to lysosome transport via multivesicular body sorting pathway / BAT3 complex binding / cellular response to arsenite ion / protein-DNA covalent cross-linking repair / Derlin-1 retrotranslocation complex / positive regulation of protein K63-linked deubiquitination / cytoplasm protein quality control / positive regulation of oxidative phosphorylation / aggresome assembly / deubiquitinase activator activity / ubiquitin-modified protein reader activity / regulation of protein localization to chromatin / mitotic spindle disassembly / cellular response to misfolded protein / VCP-NPL4-UFD1 AAA ATPase complex / ciliary transition zone / positive regulation of mitochondrial membrane potential / vesicle-fusing ATPase / K48-linked polyubiquitin modification-dependent protein binding / regulation of aerobic respiration / retrograde protein transport, ER to cytosol / stress granule disassembly / NAD+ metabolic process / ATPase complex / regulation of synapse organization / ubiquitin-specific protease binding / ciliary tip / positive regulation of ATP biosynthetic process / MHC class I protein binding / ubiquitin-like protein ligase binding / RHOH GTPase cycle / polyubiquitin modification-dependent protein binding / autophagosome maturation / endoplasmic reticulum to Golgi vesicle-mediated transport / negative regulation of hippo signaling / HSF1 activation / interstrand cross-link repair / ATP metabolic process / translesion synthesis / endoplasmic reticulum unfolded protein response / proteasomal protein catabolic process / negative regulation of protein localization to chromatin / Attachment and Entry / Protein methylation / ERAD pathway / lipid droplet / proteasome complex / viral genome replication / Josephin domain DUBs / N-glycan trimming in the ER and Calnexin/Calreticulin cycle / negative regulation of smoothened signaling pathway / macroautophagy / establishment of protein localization / Hh mutants are degraded by ERAD / positive regulation of protein-containing complex assembly / Hedgehog ligand biogenesis / Defective CFTR causes cystic fibrosis / positive regulation of non-canonical NF-kappaB signal transduction / Translesion Synthesis by POLH / ADP binding / autophagy / ABC-family proteins mediated transport / cytoplasmic stress granule / Aggrephagy / azurophil granule lumen / positive regulation of protein catabolic process / Ovarian tumor domain proteases / KEAP1-NFE2L2 pathway / positive regulation of canonical Wnt signaling pathway / double-strand break repair / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / E3 ubiquitin ligases ubiquitinate target proteins / cellular response to heat / site of double-strand break / Neddylation / secretory granule lumen / protein phosphatase binding / regulation of apoptotic process / ubiquitin-dependent protein catabolic process / ficolin-1-rich granule lumen / proteasome-mediated ubiquitin-dependent protein catabolic process / Attachment and Entry / ciliary basal body / protein ubiquitination / intracellular membrane-bounded organelle / protein domain specific binding / DNA repair / DNA damage response / ubiquitin protein ligase binding / Neutrophil degranulation / lipid binding / endoplasmic reticulum membrane / perinuclear region of cytoplasm / glutamatergic synapse / endoplasmic reticulum / ATP hydrolysis activity Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
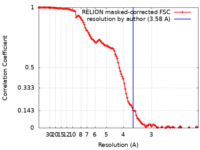
| Method | single particle reconstruction / cryo EM / Resolution: 3.58 Å | |||||||||
 Authors Authors | Pan M / Yu Y / Liu L / Zhao M | |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2021 Journal: Nat Struct Mol Biol / Year: 2021Title: Mechanistic insight into substrate processing and allosteric inhibition of human p97. Authors: Man Pan / Yuanyuan Yu / Huasong Ai / Qingyun Zheng / Yuan Xie / Lei Liu / Minglei Zhao /   Abstract: p97 processes ubiquitinated substrates and plays a central role in cellular protein homeostasis. Here, we report a series of cryo-EM structures of the substrate-engaged human p97 complex with ...p97 processes ubiquitinated substrates and plays a central role in cellular protein homeostasis. Here, we report a series of cryo-EM structures of the substrate-engaged human p97 complex with resolutions ranging from 2.9 to 3.8 Å that captured 'power-stroke'-like motions of both the D1 and D2 ATPase rings of p97. A key feature of these structures is the critical conformational changes of the intersubunit signaling (ISS) motifs, which tighten the binding of nucleotides and neighboring subunits and contribute to the spiral staircase conformation of the D1 and D2 rings. In addition, we determined the cryo-EM structure of human p97 in complex with NMS-873, a potent p97 inhibitor, at a resolution of 2.4 Å. The structures showed that NMS-873 binds at a cryptic groove in the D2 domain and interacts with the ISS motif, preventing its conformational change and thus blocking substrate translocation allosterically. | |||||||||
| History |
|
- Structure visualization
Structure visualization
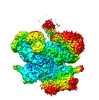
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23450.map.gz emd_23450.map.gz | 98.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23450-v30.xml emd-23450-v30.xml emd-23450.xml emd-23450.xml | 22.7 KB 22.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23450_fsc.xml emd_23450_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_23450.png emd_23450.png | 150.5 KB | ||
| Masks |  emd_23450_msk_1.map emd_23450_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-23450.cif.gz emd-23450.cif.gz | 5.9 KB | ||
| Others |  emd_23450_additional_1.map.gz emd_23450_additional_1.map.gz emd_23450_half_map_1.map.gz emd_23450_half_map_1.map.gz emd_23450_half_map_2.map.gz emd_23450_half_map_2.map.gz | 116.8 MB 98.3 MB 98.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23450 http://ftp.pdbj.org/pub/emdb/structures/EMD-23450 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23450 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23450 | HTTPS FTP |
-Related structure data
| Related structure data |  7ln6MC  7lmyC  7lmzC  7ln0C  7ln1C  7ln2C  7ln3C  7ln4C  7ln5C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23450.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23450.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.063 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_23450_msk_1.map emd_23450_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #1
| File | emd_23450_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_23450_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_23450_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human p97 in complex with Npl4/Ufd1 and K48-linked polyubiquitina...
| Entire | Name: Human p97 in complex with Npl4/Ufd1 and K48-linked polyubiquitinated Ub-Eos in the presence of ATP |
|---|---|
| Components |
|
-Supramolecule #1: Human p97 in complex with Npl4/Ufd1 and K48-linked polyubiquitina...
| Supramolecule | Name: Human p97 in complex with Npl4/Ufd1 and K48-linked polyubiquitinated Ub-Eos in the presence of ATP type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Transitional endoplasmic reticulum ATPase
| Macromolecule | Name: Transitional endoplasmic reticulum ATPase / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO / EC number: vesicle-fusing ATPase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 89.493852 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASGADSKGD DLSTAILKQK NRPNRLIVDE AINEDNSVVS LSQPKMDELQ LFRGDTVLLK GKKRREAVCI VLSDDTCSDE KIRMNRVVR NNLRVRLGDV ISIQPCPDVK YGKRIHVLPI DDTVEGITGN LFEVYLKPYF LEAYRPIRKG DIFLVRGGMR A VEFKVVET ...String: MASGADSKGD DLSTAILKQK NRPNRLIVDE AINEDNSVVS LSQPKMDELQ LFRGDTVLLK GKKRREAVCI VLSDDTCSDE KIRMNRVVR NNLRVRLGDV ISIQPCPDVK YGKRIHVLPI DDTVEGITGN LFEVYLKPYF LEAYRPIRKG DIFLVRGGMR A VEFKVVET DPSPYCIVAP DTVIHCEGEP IKREDEEESL NEVGYDDIGG CRKQLAQIKE MVELPLRHPA LFKEIGVKPP RG ILLYGPP GTGKTLIARA VANETGAFFF LINGPEIMSK LAGESESNLR KAFEEAEKNA PAIIFIDELD AIAPKREKTH GEV ERRIVS QLLTLMDGLK QRAHVIVMAA TNRPNSIDPA LRRFGRFDRE VDIGIPDATG RLEILQIHTK NMKLADDVDL EQVA NETHG HVGADLAALC SEAALQAIRK KMDLIDLEDE TIDAEVMNSL AVTMDDFRWA LSQSNPSALR ETVVEVPQVT WEDIG GLED VKRELQELVQ YPVEHPDKFL KFGMTPSKGV LFYGPPGCGK TLLAKAIANE CQANFISIKG PELLTMWFGE SEANVR EIF DKARQAAPCV LFFDQLDSIA KARGGNIGDG GGAADRVINQ ILTEMDGMST KKNVFIIGAT NRPDIIDPAI LRPGRLD QL IYIPLPDEKS RVAILKANLR KSPVAKDVDL EFLAKMTNGF SGADLTEICQ RACKLAIRES IESEIRRERE RQTNPSAM E VEEDDPVPEI RRDHFEEAMR FARRSVSDND IRKYEMFAQT LQQSRGFGSF RFPSGNQGGA GPSQGSGGGT GGSVYTEDN DDDLYG UniProtKB: Transitional endoplasmic reticulum ATPase |
-Macromolecule #2: polyubiquitinated Ub-Eos
| Macromolecule | Name: polyubiquitinated Ub-Eos / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 1.975426 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 5 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #4: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 7 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 7 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.5 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-7ln6: |
 Movie
Movie Controller
Controller







































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)