[English] 日本語
 Yorodumi
Yorodumi- EMDB-23295: Trimeric human Arginase 1 in complex with mAb1 - 2 hArg:2 mAb1 complex -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23295 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
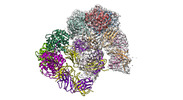
| Title | Trimeric human Arginase 1 in complex with mAb1 - 2 hArg:2 mAb1 complex | |||||||||
 Map data Map data | Full sharpened map of 2 hARG trimers bound to 2 Mab1, with better definition for one of the trimer/Fab half. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Arginase / Metalloenzyme / IMMUNE SYSTEM / HYDROLASE-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of neutrophil mediated killing of fungus / Urea cycle / negative regulation of T-helper 2 cell cytokine production / arginase / arginase activity / : / urea cycle / response to nematode / defense response to protozoan / negative regulation of type II interferon-mediated signaling pathway ...positive regulation of neutrophil mediated killing of fungus / Urea cycle / negative regulation of T-helper 2 cell cytokine production / arginase / arginase activity / : / urea cycle / response to nematode / defense response to protozoan / negative regulation of type II interferon-mediated signaling pathway / negative regulation of activated T cell proliferation / L-arginine catabolic process / negative regulation of T cell proliferation / specific granule lumen / azurophil granule lumen / manganese ion binding / adaptive immune response / innate immune response / Neutrophil degranulation / extracellular space / extracellular region / nucleus / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.15 Å | |||||||||
 Authors Authors | Gomez-Llorente Y / Scapin G | |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2021 Journal: Commun Biol / Year: 2021Title: Cryo-EM structures of inhibitory antibodies complexed with arginase 1 provide insight into mechanism of action. Authors: Rachel L Palte / Veronica Juan / Yacob Gomez-Llorente / Marc Andre Bailly / Kalyan Chakravarthy / Xun Chen / Daniel Cipriano / Ghassan N Fayad / Laurence Fayadat-Dilman / Symon Gathiaka / ...Authors: Rachel L Palte / Veronica Juan / Yacob Gomez-Llorente / Marc Andre Bailly / Kalyan Chakravarthy / Xun Chen / Daniel Cipriano / Ghassan N Fayad / Laurence Fayadat-Dilman / Symon Gathiaka / Heiko Greb / Brian Hall / Mas Handa / Mark Hsieh / Esther Kofman / Heping Lin / J Richard Miller / Nhung Nguyen / Jennifer O'Neil / Hussam Shaheen / Eric Sterner / Corey Strickland / Angie Sun / Shane Taremi / Giovanna Scapin /  Abstract: Human Arginase 1 (hArg1) is a metalloenzyme that catalyzes the hydrolysis of L-arginine to L-ornithine and urea, and modulates T-cell-mediated immune response. Arginase-targeted therapies have been ...Human Arginase 1 (hArg1) is a metalloenzyme that catalyzes the hydrolysis of L-arginine to L-ornithine and urea, and modulates T-cell-mediated immune response. Arginase-targeted therapies have been pursued across several disease areas including immunology, oncology, nervous system dysfunction, and cardiovascular dysfunction and diseases. Currently, all published hArg1 inhibitors are small molecules usually less than 350 Da in size. Here we report the cryo-electron microscopy structures of potent and inhibitory anti-hArg antibodies bound to hArg1 which form distinct macromolecular complexes that are greater than 650 kDa. With local resolutions of 3.5 Å or better we unambiguously mapped epitopes and paratopes for all five antibodies and determined that the antibodies act through orthosteric and allosteric mechanisms. These hArg1:antibody complexes present an alternative mechanism to inhibit hArg1 activity and highlight the ability to utilize antibodies as probes in the discovery and development of peptide and small molecule inhibitors for enzymes in general. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23295.map.gz emd_23295.map.gz | 137.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23295-v30.xml emd-23295-v30.xml emd-23295.xml emd-23295.xml | 23.3 KB 23.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23295.png emd_23295.png | 73.2 KB | ||
| Masks |  emd_23295_msk_1.map emd_23295_msk_1.map | 147.3 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-23295.cif.gz emd-23295.cif.gz | 6.7 KB | ||
| Others |  emd_23295_additional_1.map.gz emd_23295_additional_1.map.gz emd_23295_half_map_1.map.gz emd_23295_half_map_1.map.gz emd_23295_half_map_2.map.gz emd_23295_half_map_2.map.gz | 5.9 MB 136.5 MB 136.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23295 http://ftp.pdbj.org/pub/emdb/structures/EMD-23295 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23295 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23295 | HTTPS FTP |
-Validation report
| Summary document |  emd_23295_validation.pdf.gz emd_23295_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23295_full_validation.pdf.gz emd_23295_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_23295_validation.xml.gz emd_23295_validation.xml.gz | 13.8 KB | Display | |
| Data in CIF |  emd_23295_validation.cif.gz emd_23295_validation.cif.gz | 16.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23295 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23295 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23295 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23295 | HTTPS FTP |
-Related structure data
| Related structure data |  7lezMC  7lexC  7leyC  7lf0C  7lf1C  7lf2C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23295.map.gz / Format: CCP4 / Size: 147.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23295.map.gz / Format: CCP4 / Size: 147.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full sharpened map of 2 hARG trimers bound to 2 Mab1, with better definition for one of the trimer/Fab half. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
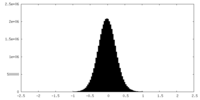
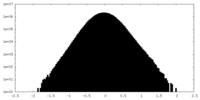
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_23295_msk_1.map emd_23295_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Map filtered at the final resolution.
| File | emd_23295_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map filtered at the final resolution. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A.
| File | emd_23295_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B.
| File | emd_23295_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Trimeric human arginase in complex with mAb1
| Entire | Name: Trimeric human arginase in complex with mAb1 |
|---|---|
| Components |
|
-Supramolecule #1: Trimeric human arginase in complex with mAb1
| Supramolecule | Name: Trimeric human arginase in complex with mAb1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 Details: 2 trimers of human arginase bound to the Fab regions of 2 mAb1 molecules |
|---|---|
| Molecular weight | Theoretical: 427 KDa |
-Supramolecule #2: mAb1
| Supramolecule | Name: mAb1 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: Arginase-1
| Supramolecule | Name: Arginase-1 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: mAb1 heavy chain
| Macromolecule | Name: mAb1 heavy chain / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 50.269605 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLVQSGAE VKKPGASVKV SCKASGYTFT NYGISWVRQA PGQGLEWMGW ISAYNGNTNY AQKLQGRVTM TTDTSTSTAY MELRSLRSD DTAVYYCARE GAYGYRSPYH NWFDPWGQGT LVTVSSAKTT APSVYPLAPV CGDTTGSSVT LGCLVKGYFP E PVTLTWNS ...String: QVQLVQSGAE VKKPGASVKV SCKASGYTFT NYGISWVRQA PGQGLEWMGW ISAYNGNTNY AQKLQGRVTM TTDTSTSTAY MELRSLRSD DTAVYYCARE GAYGYRSPYH NWFDPWGQGT LVTVSSAKTT APSVYPLAPV CGDTTGSSVT LGCLVKGYFP E PVTLTWNS GSLSSGVHTF PAVLQSDLYT LSSSVTVTSS TWPSQSITCN VAHPASSTKV DKKIEPRGPT IKPCPPCKCP AP NLLGGPS VFIFPPKIKD VLMISLSPIV TCVVVDVSED DPDVQISWFV NNVEVHTAQT QTHREDYNST LRVVSALPIQ HQD WMSGKE FKCKVNNKDL PAPIERTISK PKGSVRAPQV YVLPPPEEEM TKKQVTLTCM VTDFMPEDIY VEWTNNGKTE LNYK NTEPV LDSDGSYFMY SKLRVEKKNW VERNSYSCSV VHEGLHNHHT TKSFSRTPGK |
-Macromolecule #2: mAb1 light chain
| Macromolecule | Name: mAb1 light chain / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.488965 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: EIVMTQSPAT LSLSPGERAT LSCRASQSVS SYLAWYQQKP GQAPRLLIYD ASNRATGIPA RFSGSGSGTD FTLTISSLEP EDFAVYYCQ QHSLLPRTFG GGTKVEIKRA DAAPTVSIFP PSSEQLTSGG ASVVCFLNNF YPKDINVKWK IDGSERQNGV L NSWTDQDS ...String: EIVMTQSPAT LSLSPGERAT LSCRASQSVS SYLAWYQQKP GQAPRLLIYD ASNRATGIPA RFSGSGSGTD FTLTISSLEP EDFAVYYCQ QHSLLPRTFG GGTKVEIKRA DAAPTVSIFP PSSEQLTSGG ASVVCFLNNF YPKDINVKWK IDGSERQNGV L NSWTDQDS KDSTYSMSST LTLTKDEYER HNSYTCEATH KTSTSPIVKS FNRNEC |
-Macromolecule #3: Arginase-1
| Macromolecule | Name: Arginase-1 / type: protein_or_peptide / ID: 3 / Number of copies: 6 / Enantiomer: LEVO / EC number: arginase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 34.779879 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSAKSRTIGI IGAPFSKGQP RGGVEEGPTV LRKAGLLEKL KEQECDVKDY GDLPFADIPN DSPFQIVKNP RSVGKASEQL AGKVAEVKK NGRISLVLGG DHSLAIGSIS GHARVHPDLG VIWVDAHTDI NTPLTTTSGN LHGQPVSFLL KELKGKIPDV P GFSWVTPC ...String: MSAKSRTIGI IGAPFSKGQP RGGVEEGPTV LRKAGLLEKL KEQECDVKDY GDLPFADIPN DSPFQIVKNP RSVGKASEQL AGKVAEVKK NGRISLVLGG DHSLAIGSIS GHARVHPDLG VIWVDAHTDI NTPLTTTSGN LHGQPVSFLL KELKGKIPDV P GFSWVTPC ISAKDIVYIG LRDVDPGEHY ILKTLGIKYF SMTEVDRLGI GKVMEETLSY LLGRKKRPIH LSFDVDGLDP SF TPATGTP VVGGLTYREG LYITEEIYKT GLLSGLDIME VNPSLGKTPE EVTRTVNTAV AITLACFGLA REGNHKPIDY LNP PK UniProtKB: Arginase-1 |
-Macromolecule #4: MANGANESE (II) ION
| Macromolecule | Name: MANGANESE (II) ION / type: ligand / ID: 4 / Number of copies: 12 / Formula: MN |
|---|---|
| Molecular weight | Theoretical: 54.938 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 30 sec. |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Number grids imaged: 1 / Number real images: 1506 / Average exposure time: 6.0 sec. / Average electron dose: 45.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-7lez: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)