[English] 日本語
 Yorodumi
Yorodumi- EMDB-22777: Cryo-EM structure of the Sec complex from T. lanuginosus, Sec62 a... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22777 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the Sec complex from T. lanuginosus, Sec62 anchor domain mutant (delta anchor) | |||||||||
 Map data Map data | Unsharpened, lowpass-filtered map | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationmisfolded protein transport / Sec62/Sec63 complex / translocon complex / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / rough endoplasmic reticulum membrane / cytosol to endoplasmic reticulum transport / Ssh1 translocon complex / Sec61 translocon complex / protein-transporting ATPase activity / post-translational protein targeting to endoplasmic reticulum membrane ...misfolded protein transport / Sec62/Sec63 complex / translocon complex / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / rough endoplasmic reticulum membrane / cytosol to endoplasmic reticulum transport / Ssh1 translocon complex / Sec61 translocon complex / protein-transporting ATPase activity / post-translational protein targeting to endoplasmic reticulum membrane / SRP-dependent cotranslational protein targeting to membrane, translocation / filamentous growth / signal sequence binding / SRP-dependent cotranslational protein targeting to membrane / post-translational protein targeting to membrane, translocation / peptide transmembrane transporter activity / nuclear inner membrane / retrograde protein transport, ER to cytosol / protein transmembrane transporter activity / ERAD pathway / guanyl-nucleotide exchange factor activity / cell periphery / ribosome binding / endoplasmic reticulum membrane / structural molecule activity / endoplasmic reticulum / mitochondrion / membrane / cytosol Similarity search - Function | |||||||||
| Biological species |   Thermomyces lanuginosus (fungus) Thermomyces lanuginosus (fungus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.4 Å | |||||||||
 Authors Authors | Itskanov S / Park E | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2021 Journal: Nat Struct Mol Biol / Year: 2021Title: Stepwise gating of the Sec61 protein-conducting channel by Sec63 and Sec62. Authors: Samuel Itskanov / Katie M Kuo / James C Gumbart / Eunyong Park /  Abstract: Many proteins are transported into the endoplasmic reticulum by the universally conserved Sec61 channel. Post-translational transport requires two additional proteins, Sec62 and Sec63, but their ...Many proteins are transported into the endoplasmic reticulum by the universally conserved Sec61 channel. Post-translational transport requires two additional proteins, Sec62 and Sec63, but their functions are poorly defined. In the present study, we determined cryo-electron microscopy (cryo-EM) structures of several variants of Sec61-Sec62-Sec63 complexes from Saccharomyces cerevisiae and Thermomyces lanuginosus and show that Sec62 and Sec63 induce opening of the Sec61 channel. Without Sec62, the translocation pore of Sec61 remains closed by the plug domain, rendering the channel inactive. We further show that the lateral gate of Sec61 must first be partially opened by interactions between Sec61 and Sec63 in cytosolic and luminal domains, a simultaneous disruption of which completely closes the channel. The structures and molecular dynamics simulations suggest that Sec62 may also prevent lipids from invading the channel through the open lateral gate. Our study shows how Sec63 and Sec62 work together in a hierarchical manner to activate Sec61 for post-translational protein translocation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22777.map.gz emd_22777.map.gz | 32 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22777-v30.xml emd-22777-v30.xml emd-22777.xml emd-22777.xml | 25.3 KB 25.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22777_fsc.xml emd_22777_fsc.xml | 9.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_22777.png emd_22777.png | 32.1 KB | ||
| Masks |  emd_22777_msk_1.map emd_22777_msk_1.map | 64 MB |  Mask map Mask map | |
| Others |  emd_22777_additional_1.map.gz emd_22777_additional_1.map.gz emd_22777_half_map_1.map.gz emd_22777_half_map_1.map.gz emd_22777_half_map_2.map.gz emd_22777_half_map_2.map.gz | 59.6 MB 59.4 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22777 http://ftp.pdbj.org/pub/emdb/structures/EMD-22777 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22777 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22777 | HTTPS FTP |
-Related structure data
| Related structure data |  7kahC  7kaiC  7kajC  7kakC  7kalC  7kamC  7kanC  7kaoC  7kapC  7kaqC  7karC  7kasC  7katC  7kauC  7kb5C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22777.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22777.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened, lowpass-filtered map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.19 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_22777_msk_1.map emd_22777_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
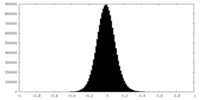
| Density Histograms |
-Additional map: Sharpened map
| File | emd_22777_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
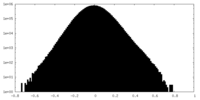
| Density Histograms |
-Half map: half-volume 1
| File | emd_22777_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-volume 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half-volume 2
| File | emd_22777_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-volume 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Endoplasmic reticulum protein-transport machinery Sec complex fro...
| Entire | Name: Endoplasmic reticulum protein-transport machinery Sec complex from T. lanuginosus |
|---|---|
| Components |
|
-Supramolecule #1: Endoplasmic reticulum protein-transport machinery Sec complex fro...
| Supramolecule | Name: Endoplasmic reticulum protein-transport machinery Sec complex from T. lanuginosus type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Anchor domain of Sec62 (residues 319-338) is replaced with the linker GGSGGSGGS |
|---|---|
| Source (natural) | Organism:   Thermomyces lanuginosus (fungus) Thermomyces lanuginosus (fungus) |
| Recombinant expression | Organism:  |
-Macromolecule #1: Protein transport channel Sec61 complex, alpha subunit (Sec61)
| Macromolecule | Name: Protein transport channel Sec61 complex, alpha subunit (Sec61) type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Thermomyces lanuginosus (fungus) Thermomyces lanuginosus (fungus) |
| Recombinant expression | Organism:  |
| Sequence | String: MSGLRFLDLI KPFTPLLPEV AAPETKVPFN QKLMWTGLTL LIFLVMSQMP LYGIVSSDTS DPLYWLRMM LASNRGTLME LGITPIISSG MVFQLLAGTH LIDVNLDLKT DRELYQTAQK L FAIILSFG QACVHVLTGL YGQPSDLGAG ICVLLIVQLV VAGLVVILLD ...String: MSGLRFLDLI KPFTPLLPEV AAPETKVPFN QKLMWTGLTL LIFLVMSQMP LYGIVSSDTS DPLYWLRMM LASNRGTLME LGITPIISSG MVFQLLAGTH LIDVNLDLKT DRELYQTAQK L FAIILSFG QACVHVLTGL YGQPSDLGAG ICVLLIVQLV VAGLVVILLD ELLQKGYGLG SG ISLFIAT NICESIVWKA FSPTTINTGR GPEFEGAIIA LFHLLLTWPD KQRALREAFY RQS LPNIMN LLATLLVFAA VIYLQGFRVE IPVKSARQRG VRGSYPVRLF YTSNMPIMLQ SALC SNVFL ISQMLYSRFS DNLLVRLLGV WEPREGSAQL HAASGIAYYM SPPLNFKEAL LDPVH TVVY ITFMLVACAL FSKTWIEVSG SSPRDVAKQL KDQGLVMAGH REQSMYKELK RVIPTA AAF GGACIGALSV ASDLLGALGS GTGILLAVTI IYGYFEMAAR EGDFGQGLRG LVPGNGS |
-Macromolecule #2: Protein transport channel Sec61 complex, beta subunit (Sbh1)
| Macromolecule | Name: Protein transport channel Sec61 complex, beta subunit (Sbh1) type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Thermomyces lanuginosus (fungus) Thermomyces lanuginosus (fungus) |
| Recombinant expression | Organism:  |
| Sequence | String: MASSGAESGS ESKSPNPGAG SGPGSASGSS AGVIRPSSPT PPGGPRAAIR RRAAADHKES LRNARPSST RAAGAGGSSG TMLKLYTDES PGLRVDPVVV LVLSLCFIFS VVGLHVIAKI T RKFSS |
-Macromolecule #3: Protein transport channel Sec61 complex, gamma subunit (Sss1)
| Macromolecule | Name: Protein transport channel Sec61 complex, gamma subunit (Sss1) type: protein_or_peptide / ID: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Thermomyces lanuginosus (fungus) Thermomyces lanuginosus (fungus) |
| Recombinant expression | Organism:  |
| Sequence | String: MSEQVQELLD IPRDFLKDGM QFIHKCQKPD RKEFKKVCQA VAIGFVAMGA IGYIVKLVHI PINNILVAG S |
-Macromolecule #4: Protein transport protein Sec63
| Macromolecule | Name: Protein transport protein Sec63 / type: protein_or_peptide / ID: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Thermomyces lanuginosus (fungus) Thermomyces lanuginosus (fungus) |
| Recombinant expression | Organism:  |
| Sequence | String: GGSGGSGGSG GSGGSMSSRE YNYDENGQFF PFFVLTLTGL VTLPLTYSLL KPPKK VEST APRIKSDFKP QHDDIIQNQK RKRLRKERRV KRAIAVVVGW AIIGYMVYLI IVTRRT APK IWDPYEILGI SRSADERAIA RRYKRLSLLY HPDKVRPDPS KNETMEMLNQ ...String: GGSGGSGGSG GSGGSMSSRE YNYDENGQFF PFFVLTLTGL VTLPLTYSLL KPPKK VEST APRIKSDFKP QHDDIIQNQK RKRLRKERRV KRAIAVVVGW AIIGYMVYLI IVTRRT APK IWDPYEILGI SRSADERAIA RRYKRLSLLY HPDKVRPDPS KNETMEMLNQ RFVELTK AY KALTDEEIRN NYLQYGHPDG KQSYSIGIAL PKLIIEEGSG KYVLMLYASL LGILLPYI V GRWWYGSQRY TREKVLAASA GNMFREYEGT MIGGPIVNAL STGEEYKEML SGPKAEEGL AKVEKKVLAL DEKILSAKDR EVLRKIDNPV RRKALALLWA YLNRIDLEDP VLNEEKYEAG SIALSLTES FTAIALAFGN LIPIIGAYRI SQCIVQAISP GSSPLLQLPY FTPKVVESVE G ADVKTHLS VQKYLDMPEE RRRSLTVGPG LLTEDQYNSA IAVAKQLPLF AISKAFFKVA GE RVVTPSS LVQLVIKGRI IPPGSTGVPD VTEKDLEDID PDEADVNAII GRKGATKPSG KSG DENDGD RVQPPLAHAP YLPRDHPPRW HIFLADAKQG KIAVPPFTFT TFDKPIFDEQ GKPT FNMQT LRMQFQAPPQ VGNFSFVLHM ISDSYMGFDV KQEITLQVED PSKAAVLQEE DDISE PDED SIAGQMQALK TGVPPKKKKV VESDDDESDT EGDEEDTSET DTETDTDEEG SGTGEN LYF Q |
-Macromolecule #5: Protein transport protein Sec66/Sec71
| Macromolecule | Name: Protein transport protein Sec66/Sec71 / type: protein_or_peptide / ID: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Thermomyces lanuginosus (fungus) Thermomyces lanuginosus (fungus) |
| Recombinant expression | Organism:  |
| Sequence | String: MDWLTLVVPF AYLGVLIGCL ATFSSLYRRR KAAKAASLEP WFPPHLQRDI YHSLLHLDQQ QQNEKKTRV PETVLKAALL RRAAEDIKRV MAIREQKQAL ALLLQRGSVG DELWQRFLRA E KEMEDEVR DVVAEANSYA PNWGQVIFQS AREMDANATY RARMEEYQAT ...String: MDWLTLVVPF AYLGVLIGCL ATFSSLYRRR KAAKAASLEP WFPPHLQRDI YHSLLHLDQQ QQNEKKTRV PETVLKAALL RRAAEDIKRV MAIREQKQAL ALLLQRGSVG DELWQRFLRA E KEMEDEVR DVVAEANSYA PNWGQVIFQS AREMDANATY RARMEEYQAT VAEERAWWDK KR ASIQEGF MKELDAEKER PATAASTATN TTSTTSDDDA VLVEAEKEGT SSPAPGKKKK KGK KGS |
-Macromolecule #6: Protein transport protein Sec72
| Macromolecule | Name: Protein transport protein Sec72 / type: protein_or_peptide / ID: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Thermomyces lanuginosus (fungus) Thermomyces lanuginosus (fungus) |
| Recombinant expression | Organism:  |
| Sequence | String: MSSDLDTYTH YPLHLDPSSK AVSLATTEGQ TPAQTEAVEA ELQQLNALHR SLISLDPPNV PPPPLPINP KRSAQITKLK ETANTAYKRG NHGEAVRLYS YAIEMAAGRP GWEPVNLARE E LSGLYANR AQAHMAQQMW PEGWVDAKCS VESKPVGNAK GWWRGGKCLV ...String: MSSDLDTYTH YPLHLDPSSK AVSLATTEGQ TPAQTEAVEA ELQQLNALHR SLISLDPPNV PPPPLPINP KRSAQITKLK ETANTAYKRG NHGEAVRLYS YAIEMAAGRP GWEPVNLARE E LSGLYANR AQAHMAQQMW PEGWVDAKCS VESKPVGNAK GWWRGGKCLV EMGRYDEARA WI EQALGIE GPASDGGKEL AALLEEIKAG SQRRQGS |
-Macromolecule #7: Protein transport protein Sec62
| Macromolecule | Name: Protein transport protein Sec62 / type: protein_or_peptide / ID: 7 Details: T. lanuginosus Sec62 with residues 319-338 replaced with the linker GGSGGSGGS Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Thermomyces lanuginosus (fungus) Thermomyces lanuginosus (fungus) |
| Recombinant expression | Organism:  |
| Sequence | String: MAAPPPGMTP QQFAALQQHM QQQIAAEAAK RGMTVEEFSK MQREQLNAEA AKAGMTPEQY INQLRMRALQ QRAAMQQQMQ QQQQQGGQGQ TQGQGQGQGQ QGQGQPQVQH VQHVQQQVSV NPNAPPNPKA IALAKWLRSQ NLKARTCILD GQRREMFKVK RALRALESPE ...String: MAAPPPGMTP QQFAALQQHM QQQIAAEAAK RGMTVEEFSK MQREQLNAEA AKAGMTPEQY INQLRMRALQ QRAAMQQQMQ QQQQQGGQGQ TQGQGQGQGQ QGQGQPQVQH VQHVQQQVSV NPNAPPNPKA IALAKWLRSQ NLKARTCILD GQRREMFKVK RALRALESPE YQKAAAKNKL LPPVTDRASA ENAFKLLPLS FLALRVSKVS SNYNKGKRVK GLWTVKVEQH QDTDPMTHYV WLYEGPQWKQ KALAAAFVIG IFAIVLFPLW PIMLRQGVWY LSVGMLGLLG LFFALAIVRL ILFCVTVFVV PPGGSGGSGG SKKKKPKKAK AAVSKSQEKG AAPTTAAPEA PTATTTSSEA QPSSSSGTAS KRNLAASVED AEEGS |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 49.1 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 42017 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)