[English] 日本語
 Yorodumi
Yorodumi- EMDB-22604: Human serine palmitoyltransferase complex SPTLC1/SPLTC2/ssSPTa/OR... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22604 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human serine palmitoyltransferase complex SPTLC1/SPLTC2/ssSPTa/ORMDL3, class 2 | |||||||||
 Map data Map data | Sharpened_map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Sphingolipid / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsphinganine biosynthetic process / regulation of fat cell apoptotic process / negative regulation of ceramide biosynthetic process / sphingomyelin biosynthetic process / intracellular sphingolipid homeostasis / serine palmitoyltransferase complex / serine C-palmitoyltransferase activity / serine C-palmitoyltransferase / ceramide metabolic process / sphingosine biosynthetic process ...sphinganine biosynthetic process / regulation of fat cell apoptotic process / negative regulation of ceramide biosynthetic process / sphingomyelin biosynthetic process / intracellular sphingolipid homeostasis / serine palmitoyltransferase complex / serine C-palmitoyltransferase activity / serine C-palmitoyltransferase / ceramide metabolic process / sphingosine biosynthetic process / sphingolipid metabolic process / sphingolipid biosynthetic process / Sphingolipid de novo biosynthesis / regulation of smooth muscle contraction / ceramide biosynthetic process / positive regulation of lipophagy / negative regulation of B cell apoptotic process / motor behavior / adipose tissue development / specific granule membrane / myelination / positive regulation of autophagy / secretory granule membrane / positive regulation of protein localization to nucleus / intracellular protein localization / pyridoxal phosphate binding / Neutrophil degranulation / endoplasmic reticulum membrane / endoplasmic reticulum / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Wang Y / Niu Y | |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2021 Journal: Nat Struct Mol Biol / Year: 2021Title: Structural insights into the regulation of human serine palmitoyltransferase complexes. Authors: Yingdi Wang / Yiming Niu / Zhe Zhang / Kenneth Gable / Sita D Gupta / Niranjanakumari Somashekarappa / Gongshe Han / Hongtu Zhao / Alexander G Myasnikov / Ravi C Kalathur / Teresa M Dunn / Chia-Hsueh Lee /   Abstract: Sphingolipids are essential lipids in eukaryotic membranes. In humans, the first and rate-limiting step of sphingolipid synthesis is catalyzed by the serine palmitoyltransferase holocomplex, which ...Sphingolipids are essential lipids in eukaryotic membranes. In humans, the first and rate-limiting step of sphingolipid synthesis is catalyzed by the serine palmitoyltransferase holocomplex, which consists of catalytic components (SPTLC1 and SPTLC2) and regulatory components (ssSPTa and ORMDL3). However, the assembly, substrate processing and regulation of the complex are unclear. Here, we present 8 cryo-electron microscopy structures of the human serine palmitoyltransferase holocomplex in various functional states at resolutions of 2.6-3.4 Å. The structures reveal not only how catalytic components recognize the substrate, but also how regulatory components modulate the substrate-binding tunnel to control enzyme activity: ssSPTa engages SPTLC2 and shapes the tunnel to determine substrate specificity. ORMDL3 blocks the tunnel and competes with substrate binding through its amino terminus. These findings provide mechanistic insights into sphingolipid biogenesis governed by the serine palmitoyltransferase complex. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22604.map.gz emd_22604.map.gz | 157.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22604-v30.xml emd-22604-v30.xml emd-22604.xml emd-22604.xml | 20.2 KB 20.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22604.png emd_22604.png | 82.3 KB | ||
| Filedesc metadata |  emd-22604.cif.gz emd-22604.cif.gz | 7.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22604 http://ftp.pdbj.org/pub/emdb/structures/EMD-22604 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22604 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22604 | HTTPS FTP |
-Related structure data
| Related structure data |  7k0nMC  7k0iC  7k0jC  7k0kC  7k0lC  7k0mC  7k0oC  7k0pC  7k0qC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_22604.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22604.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened_map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.005 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
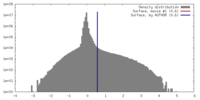
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : human serine palmitoyltransferase complexes
| Entire | Name: human serine palmitoyltransferase complexes |
|---|---|
| Components |
|
-Supramolecule #1: human serine palmitoyltransferase complexes
| Supramolecule | Name: human serine palmitoyltransferase complexes / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Serine palmitoyltransferase 1
| Macromolecule | Name: Serine palmitoyltransferase 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: serine C-palmitoyltransferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 52.80677 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MATATEQWVL VEMVQALYEA PAYHLILEGI LILWIIRLLF SKTYKLQERS DLTVKEKEEL IEEWQPEPLV PPVPKDHPAL NYNIVSGPP SHKTVVNGKE CINFASFNFL GLLDNPRVKA AALASLKKYG VGTCGPRGFY GTFDVHLDLE DRLAKFMKTE E AIIYSYGF ...String: MATATEQWVL VEMVQALYEA PAYHLILEGI LILWIIRLLF SKTYKLQERS DLTVKEKEEL IEEWQPEPLV PPVPKDHPAL NYNIVSGPP SHKTVVNGKE CINFASFNFL GLLDNPRVKA AALASLKKYG VGTCGPRGFY GTFDVHLDLE DRLAKFMKTE E AIIYSYGF ATIASAIPAY SKRGDIVFVD RAACFAIQKG LQASRSDIKL FKHNDMADLE RLLKEQEIED QKNPRKARVT RR FIVVEGL YMNTGTICPL PELVKLKYKY KARIFLEESL SFGVLGEHGR GVTEHYGINI DDIDLISANM ENALASIGGF CCG RSFVID HQRLSGQGYC FSASLPPLLA AAAIEALNIM EENPGIFAVL KEKCGQIHKA LQGISGLKVV GESLSPAFHL QLEE STGSR EQDVRLLQEI VDQCMNRSIA LTQARYLEKE EKCLPPPSIR VVVTVEQTEE ELERAASTIK EVAQAVLL UniProtKB: Serine palmitoyltransferase 1 |
-Macromolecule #2: Serine palmitoyltransferase 2
| Macromolecule | Name: Serine palmitoyltransferase 2 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO / EC number: serine C-palmitoyltransferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 63.232277 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MRPEPGGCCC RRTVRANGCV ANGEVRNGYV RSSAAAAAAA AAGQIHHVTQ NGGLYKRPFN EAFEETPMLV AVLTYVGYGV LTLFGYLRD FLRYWRIEKC HHATEREEQK DFVSLYQDFE NFYTRNLYMR IRDNWNRPIC SVPGARVDIM ERQSHDYNWS F KYTGNIIK ...String: MRPEPGGCCC RRTVRANGCV ANGEVRNGYV RSSAAAAAAA AAGQIHHVTQ NGGLYKRPFN EAFEETPMLV AVLTYVGYGV LTLFGYLRD FLRYWRIEKC HHATEREEQK DFVSLYQDFE NFYTRNLYMR IRDNWNRPIC SVPGARVDIM ERQSHDYNWS F KYTGNIIK GVINMGSYNY LGFARNTGSC QEAAAKVLEE YGAGVCSTRQ EIGNLDKHEE LEELVARFLG VEAAMAYGMG FA TNSMNIP ALVGKGCLIL SDELNHASLV LGARLSGATI RIFKHNNMQS LEKLLKDAIV YGQPRTRRPW KKILILVEGI YSM EGSIVR LPEVIALKKK YKAYLYLDEA HSIGALGPTG RGVVEYFGLD PEDVDVMMGT FT(LLP)SFGASGG YIGGKKELID YLRTHSHSA VYATSLSPPV VEQIITSMKC IMGQDGTSLG KECVQQLAEN TRYFRRRLKE MGFIIYGNED SPVVPLMLYM P AKIGAFGR EMLKRNIGVV VVGFPATPII ESRARFCLSA AHTKEILDTA LKEIDEVGDL LQLKYSRHRL VPLLDRPFDE TT YEETED UniProtKB: Serine palmitoyltransferase 2 |
-Macromolecule #3: Serine palmitoyltransferase small subunit A
| Macromolecule | Name: Serine palmitoyltransferase small subunit A / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 8.471095 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAGMALARAW KQMSWFYYQY LLVTALYMLE PWERTVFNSM LVSIVGMALY TGYVFMPQHI MAILHYFEIV Q UniProtKB: Serine palmitoyltransferase small subunit A |
-Macromolecule #4: ORM1-like protein 3
| Macromolecule | Name: ORM1-like protein 3 / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 17.512594 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MNVGTAHSEV NPNTRVMNSR GIWLSYVLAI GLLHIVLLSI PFVSVPVVWT LTNLIHNMGM YIFLHTVKGT PFETPDQGKA RLLTHWEQM DYGVQFTASR KFLTITPIVL YFLTSFYTKY DQIHFVLNTV SLMSVLIPKL PQLHGVRIFG INKY UniProtKB: ORM1-like protein 3 |
-Macromolecule #5: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 5 / Number of copies: 16 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-POV: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 72.53 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)