+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22427 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human HUWE1 | ||||||||||||||||||
 Map data Map data | Cryo-EM structure of human HUWE1 | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Ubiquitin / Quality Control / E3 ligase / protein degradation / TRANSFERASE | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of peroxisome proliferator activated receptor signaling pathway / histone ubiquitin ligase activity / negative regulation of mitochondrial fusion / protein branched polyubiquitination / positive regulation of type 2 mitophagy / HECT-type E3 ubiquitin transferase / : / ubiquitin-ubiquitin ligase activity / Golgi organization / protein monoubiquitination ...negative regulation of peroxisome proliferator activated receptor signaling pathway / histone ubiquitin ligase activity / negative regulation of mitochondrial fusion / protein branched polyubiquitination / positive regulation of type 2 mitophagy / HECT-type E3 ubiquitin transferase / : / ubiquitin-ubiquitin ligase activity / Golgi organization / protein monoubiquitination / protein K48-linked ubiquitination / positive regulation of protein ubiquitination / circadian regulation of gene expression / base-excision repair / protein polyubiquitination / ubiquitin-protein transferase activity / ubiquitin protein ligase activity / Antigen processing: Ubiquitination & Proteasome degradation / ubiquitin-dependent protein catabolic process / secretory granule lumen / ficolin-1-rich granule lumen / proteasome-mediated ubiquitin-dependent protein catabolic process / membrane fusion / cell differentiation / positive regulation of canonical NF-kappaB signal transduction / Golgi membrane / Neutrophil degranulation / mitochondrion / DNA binding / RNA binding / extracellular exosome / extracellular region / nucleoplasm / nucleus / membrane / cytosol / cytoplasm Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
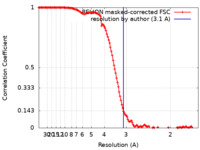
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | ||||||||||||||||||
 Authors Authors | Hunkeler M / Fischer ES | ||||||||||||||||||
| Funding support |  United States, United States,  Switzerland, 5 items Switzerland, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2021 Journal: Mol Cell / Year: 2021Title: Solenoid architecture of HUWE1 contributes to ligase activity and substrate recognition. Authors: Moritz Hunkeler / Cyrus Y Jin / Michelle W Ma / Julie K Monda / Daan Overwijn / Eric J Bennett / Eric S Fischer /  Abstract: HECT ubiquitin ligases play essential roles in metazoan development and physiology. The HECT ligase HUWE1 is central to the cellular stress response by mediating degradation of key death or survival ...HECT ubiquitin ligases play essential roles in metazoan development and physiology. The HECT ligase HUWE1 is central to the cellular stress response by mediating degradation of key death or survival factors, including Mcl1, p53, DDIT4, and Myc. Although mutations in HUWE1 and related HECT ligases are widely implicated in human disease, our molecular understanding remains limited. Here we present a comprehensive investigation of full-length HUWE1, deepening our understanding of this class of enzymes. The N-terminal ∼3,900 amino acids of HUWE1 are indispensable for proper ligase function, and our cryo-EM structures of HUWE1 offer a complete molecular picture of this large HECT ubiquitin ligase. HUWE1 forms an alpha solenoid-shaped assembly with a central pore decorated with protein interaction modules. Structures of HUWE1 variants linked to neurodevelopmental disorders as well as of HUWE1 bound to a model substrate link the functions of this essential enzyme to its three-dimensional organization. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22427.map.gz emd_22427.map.gz | 15.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22427-v30.xml emd-22427-v30.xml emd-22427.xml emd-22427.xml | 29.7 KB 29.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22427_fsc.xml emd_22427_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_22427.png emd_22427.png | 64.6 KB | ||
| Masks |  emd_22427_msk_1.map emd_22427_msk_1.map | 184 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-22427.cif.gz emd-22427.cif.gz | 9.4 KB | ||
| Others |  emd_22427_additional_1.map.gz emd_22427_additional_1.map.gz emd_22427_additional_2.map.gz emd_22427_additional_2.map.gz emd_22427_half_map_1.map.gz emd_22427_half_map_1.map.gz emd_22427_half_map_2.map.gz emd_22427_half_map_2.map.gz | 163.1 MB 9.4 MB 145.1 MB 145.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22427 http://ftp.pdbj.org/pub/emdb/structures/EMD-22427 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22427 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22427 | HTTPS FTP |
-Validation report
| Summary document |  emd_22427_validation.pdf.gz emd_22427_validation.pdf.gz | 777.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_22427_full_validation.pdf.gz emd_22427_full_validation.pdf.gz | 776.8 KB | Display | |
| Data in XML |  emd_22427_validation.xml.gz emd_22427_validation.xml.gz | 20.9 KB | Display | |
| Data in CIF |  emd_22427_validation.cif.gz emd_22427_validation.cif.gz | 26.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22427 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22427 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22427 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22427 | HTTPS FTP |
-Related structure data
| Related structure data |  7jq9MC  7mopC  7mwdC  7mweC  7mwfC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22427.map.gz / Format: CCP4 / Size: 184 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22427.map.gz / Format: CCP4 / Size: 184 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of human HUWE1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.825 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_22427_msk_1.map emd_22427_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: main map postprocessed with deepEMhancer
| File | emd_22427_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map postprocessed with deepEMhancer | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: main map blurred to B=10A2
| File | emd_22427_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map blurred to B=10A2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 2
| File | emd_22427_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 2
| File | emd_22427_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : E3 ubiquitin-protein ligase HUWE1
| Entire | Name: E3 ubiquitin-protein ligase HUWE1 |
|---|---|
| Components |
|
-Supramolecule #1: E3 ubiquitin-protein ligase HUWE1
| Supramolecule | Name: E3 ubiquitin-protein ligase HUWE1 / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all / Details: full length, crosslinked with BS3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 480 KDa |
-Macromolecule #1: E3 ubiquitin-protein ligase HUWE1
| Macromolecule | Name: E3 ubiquitin-protein ligase HUWE1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: HECT-type E3 ubiquitin transferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 486.409531 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDYKDDDDKL AAANSSIDLI STSLYKKAGF KGTNSVDMKV DRTKLKKTPT EAPADCRALI DKLKVCNDEQ LLLELQQIKT WNIGKCELY HWVDLLDRFD GILADAGQTV ENMSWMLVCD RPEREQLKML LLAVLNFTAL LIEYSFSRHL YSSIEHLTTL L ASSDMQVV ...String: MDYKDDDDKL AAANSSIDLI STSLYKKAGF KGTNSVDMKV DRTKLKKTPT EAPADCRALI DKLKVCNDEQ LLLELQQIKT WNIGKCELY HWVDLLDRFD GILADAGQTV ENMSWMLVCD RPEREQLKML LLAVLNFTAL LIEYSFSRHL YSSIEHLTTL L ASSDMQVV LAVLNLLYVF SKRSNYITRL GSDKRTPLLT RLQHLAESWG GKENGFGLAE CCRDLHMMKY PPSATTLHFE FY ADPGAEV KIEKRTTSNT LHYIHIEQLD KISESPSEIM ESLTKMYSIP KDKQMLLFTH IRLAHGFSNH RKRLQAVQAR LHA ISILVY SNALQESANS ILYNGLIEEL VDVLQITDKQ LMEIKAASLR TLTSIVHLER TPKLSSIIDC TGTASYHGFL PVLV RNCIQ AMIDPSMDPY PHQFATALFS FLYHLASYDA GGEALVSCGM MEALLKVIKF LGDEQDQITF VTRAVRVVDL ITNLD MAAF QSHSGLSIFI YRLEHEVDLC RKECPFVIKP KIQRPNTTQE GEEMETDMDG VQCIPQRAAL LKSMLNFLKK AIQDPA FSD GIRHVMDGSL PTSLKHIISN AEYYGPSLFL LATEVVTVFV FQEPSLLSSL QDNGLTDVML HALLIKDVPA TREVLGS LP NVFSALCLNA RGLQSFVQCQ PFERLFKVLL SPDYLPAMRR RRSSDPLGDT ASNLGSAVDE LMRHQPTLKT DATTAIIK L LEEICNLGRD PKYICQKPSI QKADGTATAP PPRSNHAAEE ASSEDEEEEE VQAMQSFNST QQNETEPNQQ VVGTEERIP IPLMDYILNV MKFVESILSN NTTDDHCQEF VNQKGLLPLV TILGLPNLPI DFPTSAACQA VAGVCKSILT LSHEPKVLQE GLLQLDSIL SSLEPLHRPI ESPGGSVLLR ELACAGNVAD ATLSAQATPL LHALTAAHAY IMMFVHTCRV GQSEIRSISV N QWGSQLGL SVLSKLSQLY CSLVWESTVL LSLCTPNSLP SGCEFGQADM QKLVPKDEKA GTTQGGKRSD GEQDGAAGSM DA STQGLLE GIGLDGDTLA PMETDEPTAS DSKGKSKITP AMAARIKQIK PLLSASSRLG RALAELFGLL VKLCVGSPVR QRR SHHAAS TTTAPTPAAR STASALTKLL TKGLSWQPPP YTPTPRFRLT FFICSVGFTS PMLFDERKYP YHLMLQKFLC SGGH NALFE TFNWALSMGG KVPVSEGLEH SDLPDGTGEF LDAWLMLVEK MVNPTTVLES PHSLPAKLPG GVQNFPQFSA LRFLV VTQK AAFTCIKNLW NRKPLKVYGG RMAESMLAIL CHILRGEPVI RERLSKEKEG SRGEEDTGQE EGGSRREPQV NQQQLQ QLM DMGFTREHAM EALLNTSTME QATEYLLTHP PPIMGGVVRD LSMSEEDQMM RAIAMSLGQD IPMDQRAESP EEVACRK EE EERKAREKQE EEEAKCLEKF QDADPLEQDE LHTFTDTMLP GCFHLLDELP DTVYRVCDLI MTAIKRNGAD YRDMILKQ V VNQVWEAADV LIKAALPLTT SDTKTVSEWI SQMATLPQAS NLATRILLLT LLFEELKLPC AWVVESSGIL NVLIKLLEV VQPCLQAAKE QKEVQTPKWI TPVLLLIDFY EKTAISSKRR AQMTKYLQSN SNNWRWFDDR SGRWCSYSAS NNSTIDSAWK SGETSVRFT AGRRRYTVQF TTMVQVNEET GNRRPVMLTL LRVPRLNKNS KNSNGQELEK TLEESKEMDI KRKENKGNDT P LALESTNT EKETSLEETK IGEILIQGLT EDMVTVLIRA CVSMLGVPVD PDTLHATLRL CLRLTRDHKY AMMFAELKST RM ILNLTQS SGFNGFTPLV TLLLRHIIED PCTLRHTMEK VVRSAATSGA GSTTSGVVSG SLGSREINYI LRVLGPAACR NPD IFTEVA NCCIRIALPA PRGSGTASDD EFENLRIKGP NAVQLVKTTP LKPSPLPVIP DTIKEVIYDM LNALAAYHAP EEAD KSDPK PGVMTQEVGQ LLQDMGDDVY QQYRSLTRQS SDFDTQSGFS INSQVFAADG ASTETSASGT SQGEASTPEE SRDGK KDKE GDRASEEGKQ KGKGSKPLMP TSTILRLLAE LVRSYVGIAT LIANYSYTVG QSELIKEDCS VLAFVLDHLL PHTQNA EDK DTPALARLFL ASLAAAGSGT DAQVALVNEV KAALGRALAM AESTEKHARL QAVMCIISTI MESCPSTSSF YSSATAK TQ HNGMNNIIRL FLKKGLVNDL ARVPHSLDLS SPNMANTVNA ALKPLETLSR IVNQPSSLFG SKSASSKNKS EQDAQGAS Q DSSSNQQDPG EPGEAEVQEE DHDVTQTEVA DGDIMDGEAE TDSVVIAGQP EVLSSQEMQV ENELEDLIDE LLERDGGSG NSTIIVSRSG EDESQEDVLM DEAPSNLSQA STLQANREDS MNILDPEDEE EHTQEEDSSG SNEDEDDSQD EEEEEEEDEE DDQEDDEGE EGDEDDDDDG SEMELDEDYP DMNASPLVRF ERFDREDDLI IEFDNMFSSA TDIPPSPGNI PTTHPLMVRH A DHSSLTLG SGSSTTRLTQ GIGRSQRTLR QLTANTGHTI HVHYPGNRQP NPPLILQRLL GPSAAADILQ LSSSLPLQSR GR ARLLVGN DDVHIIARSD DELLDDFFHD QSTATSQAGT LSSIPTALTR WTEECKVLDA ESMHDCVSVV KVSIVNHLEF LRD EELEER REKRRKQLAE EETKITDKGK EDKENRDQSA QCTASKSNDS TEQNLSDGTP MPDSYPTTPS STDAATSESK ETLG TLQSS QQQPTLPTPP ALGEVPQELQ SPAGEGGSST QLLMPVEPEE LGPTRPSGEA ETTQMELSPA PTITSLSPER AEDSD ALTA VSSQLEGSPM DTSSLASCTL EEAVGDTSAA GSSEQPRAGS STPGDAPPAV AEVQGRSDGS GESAQPPEDS SPPASS ESS STRDSAVAIS GADSRGILEE PLPSTSSEEE DPLAGISLPE GVDPSFLAAL PDDIRREVLQ NQLGIRPPTR TAPSTNS SA PAVVGNPGVT EVSPEFLAAL PPAIQEEVLA QQRAEQQRRE LAQNASSDTP MDPVTFIQTL PSDLRRSVLE DMEDSVLA V MPPDIAAEAQ ALRREQEARQ RQLMHERLFG HSSTSALSAI LRSPAFTSRL SGNRGVQYTR LAVQRGGTFQ MGGSSSHNR PSGSNVDTLL RLRGRLLLDH EALSCLLVLL FVDEPKLNTS RLHRVLRNLC YHAQTRHWVI RSLLSILQRS SESELCIETP KLTTSEEKG KKSSKSCGSS SHENRPLDLL HKMESKSSNQ LSWLSVSMDA ALGCRTNIFQ IQRSGGRKHT EKHASGGSTV H IHPQAAPV VCRHVLDTLI QLAKVFPSHF TQQRTKETNC ESDRERGNKA CSPCSSQSSS SGICTDFWDL LVKLDNMNVS RK GKNSVKS VPVSAGGEGE TSPYSLEASP LGQLMNMLSH PVIRRSSLLT EKLLRLLSLI SIALPENKVS EAQANSGSGA SST TTATST TSTTTTTAAS TTPTPPTAPT PVTSAPALVA ATAISTIVVA ASTTVTTPTT ATTTVSISPT TKGSKSPAKV SDGG SSSTD FKMVSSGLTE NQLQLSVEVL TSHSCSEEGL EDAANVLLQL SRGDSGTRDT VLKLLLNGAR HLGYTLCKQI GTLLA ELRE YNLEQQRRAQ CETLSPDGLP EEQPQTTKLK GKMQSRFDMA ENVVIVASQK RPLGGRELQL PSMSMLTSKT STQKFF LRV LQVIIQLRDD TRRANKKAKQ TGRLGSSGLG SASSIQAAVR QLEAEADAII QMVREGQRAR RQQQAATSES SQSEASV RR EESPMDVDQP SPSAQDTQSI ASDGTPQGEK EKEERPPELP LLSEQLSLDE LWDMLGECLK ELEESHDQHA VLVLQPAV E AFFLVHATER ESKPPVRDTR ESQLAHIKDE PPPLSPAPLT PATPSSLDPF FSREPSSMHI SSSLPPDTQK FLRFAETHR TVLNQILRQS TTHLADGPFA VLVDYIRVLD FDVKRKYFRQ ELERLDEGLR KEDMAVHVRR DHVFEDSYRE LHRKSPEEMK NRLYIVFEG EEGQDAGGLL REWYMIISRE MFNPMYALFR TSPGDRVTYT INPSSHCNPN HLSYFKFVGR IVAKAVYDNR L LECYFTRS FYKHILGKSV RYTDMESEDY HFYQGLVYLL ENDVSTLGYD LTFSTEVQEF GVCEVRDLKP NGANILVTEE NK KEYVHLV CQMRMTGAIR KQLAAFLEGF YEIIPKRLIS IFTEQELELL ISGLPTIDID DLKSNTEYHK YQSNSIQIQW FWR ALRSFD QADRAKFLQF VTGTSKVPLQ GFAALEGMNG IQKFQIHRDD RSTDRLPSAH TCFNQLDLPA YESFEKLRHM LLLA IQECS EGFGLA UniProtKB: E3 ubiquitin-protein ligase HUWE1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.9 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| |||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Support film - Film thickness: 12 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 283 K / Instrument: LEICA EM GP Details: CHAPSO detergent added to final conc. of 0.8 mM. Sample applied twice.. | |||||||||
| Details | Sample crosslinked with BS3. Monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Details | Data collection in counting mode, using multi-shot scheme (4 holes per stage position, 3 movies per hole) |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 10390 / Average exposure time: 2.4 sec. / Average electron dose: 45.68 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -2.5 µm / Nominal defocus min: -0.8 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 60.88 / Target criteria: CC |
|---|---|
| Output model |  PDB-7jq9: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)